To stabilize the global temperature and mitigate climate change, the emission of anthropogenic greenhouse gases will have to be greatly reduced. To make it possible, the energy sector will have to transfer from fossil energy to environmentally friendly and carbon neutral sources.1
Solar energy exists in abundance. In roughly 90 minutes, the solar energy that reaches the earth equals the consumption of all human societies globally during one year.2 Only a fraction of this energy is captured today, and photovoltaic (PV) modules account for a marginal part of the electricity production worldwide, around 1.8% at the end of 2016. In recent years, however, the sector has been growing exponentially at a rapid rate, which means that the ability to increase efficiency and lifespan of PV modules is interesting from an energy perspective.3
PV modules consist of a number of interconnected PV-cells, embedded in an encapsulant and a protective cover glass on the top. One of the issues facing the PV modules available today is the degradation of their encapsulant, which most often consists of ethylene vinyl acetate (EVA).
It is damaged by UV radiation with wavelengths below 350 nm. The UV radiation makes the encapsulant degrade and acquire a yellow and eventually brown hue, which reduces the efficiency of the PV modules.4,5
Developing the cover glass has become increasingly important as the share of cost for the cover glass is high.6 The cover glass7,8 has several important functionalities, e.g., providing optimal light capture, rigidity, mechanical protection, and chemical protection. Optimal light capture depends on the optical properties of the cover glass, such as absorption and reflection. The latter comprises the largest part, about 8% for a typical flat glass, which can be minimized by employing antireflective coatings.9
The optical properties of flat glass10,11 are affected by the presence of iron impurities in the glass melt as the iron in the glass increases the absorption of light in the glass in the UV-Vis region of the electromagnetic spectrum. Iron can be used as a colorant of glass, giving the glass a green tint.12 In some cases, this is a positive feature, e.g., when UV-protection is needed in beer and champagne bottles.13 In other cases, as with PV-modules where transparency is coveted,14 the iron in the glass is considered as a contaminant. In these cases, low-iron glass, where measures have been taken to reduce the iron in the glass, is frequently used.
In the case of cover glass for PV modules, the trend has been to use low-iron glass to increase transmitted light.8 A drawback to this type of glass is that a larger amount of high-energy UV radiation is transmitted, which is harmful to the encapsulation material EVA that is used in most PV modules today.15 When UV radiation below 350 nm reaches the PV module, both the semiconductor material16 and the laminate5,17 are degraded. The degradation of the EVA laminate is the major reason for the annual degradation of 0.6–2.5%.17,18 As a result of the UV radiation, EVA degrades and loses some of its high transmissivity as it gets a yellow/brown hue and eventually starts to delaminate, letting moisture into the PV modules, which leads to failure of the PV module.5
In the current study we have investigated float glass coated with ZnO and TiO2 thin films by spray pyrolysis of organometallic compounds of zinc and titanium (Table 1 and 2).
Results
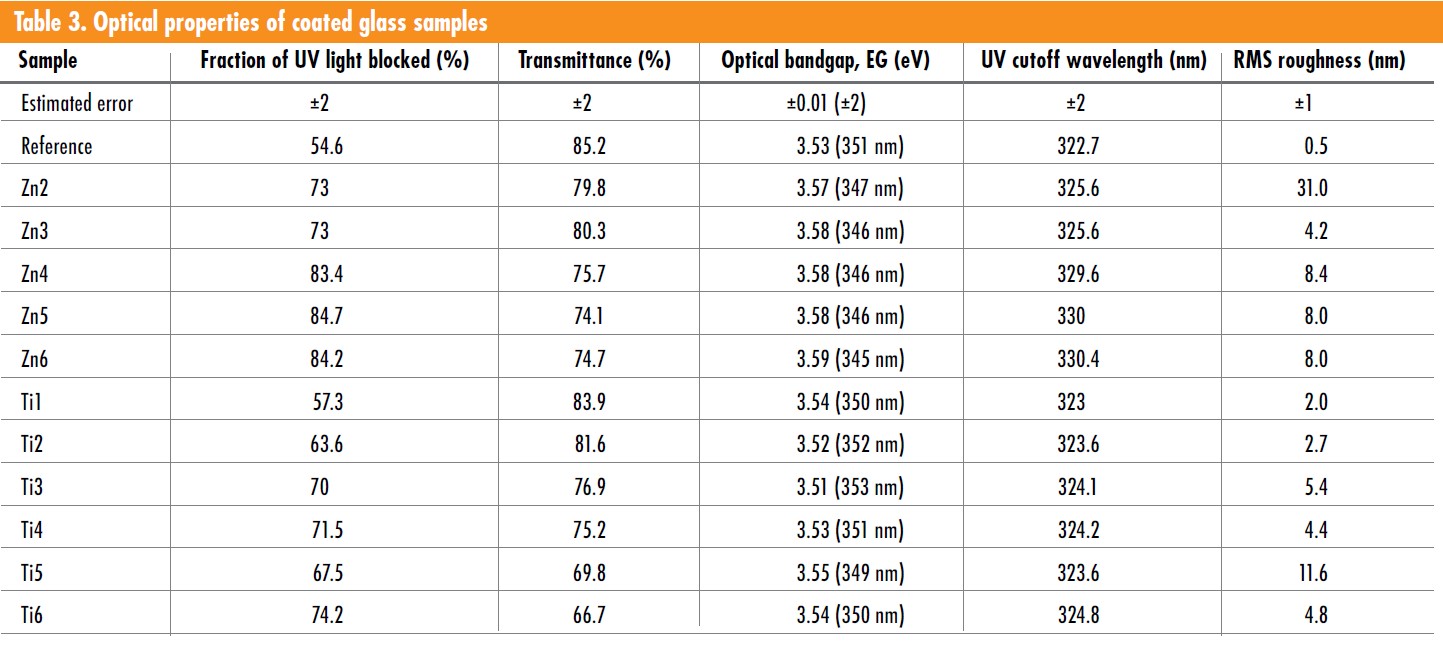
Glass coated with ZnO showed a trend to shift the UV-cutoff to longer wavelength as well as lowering the optical band gap of the coated glass sample. The major reason for this is likely to be caused by tetrahedrally coordinated Fe3+ having an absorption peak at about 380 nm but also being sensitized by the presence of the ZnO coating. Such a trend is less clear for the samples coated with TiO2.
Both sample series showed a significant increase in total reflection for the normal incident light due to the higher refractive index of the thin film oxide coatings (Figure 1a). However, the increase in diffuse reflection was significantly lower, less than 4% (Figure 1b); this is an advantage for application on the cover glass of PV-modules where most of the incoming light will be of diffuse character.
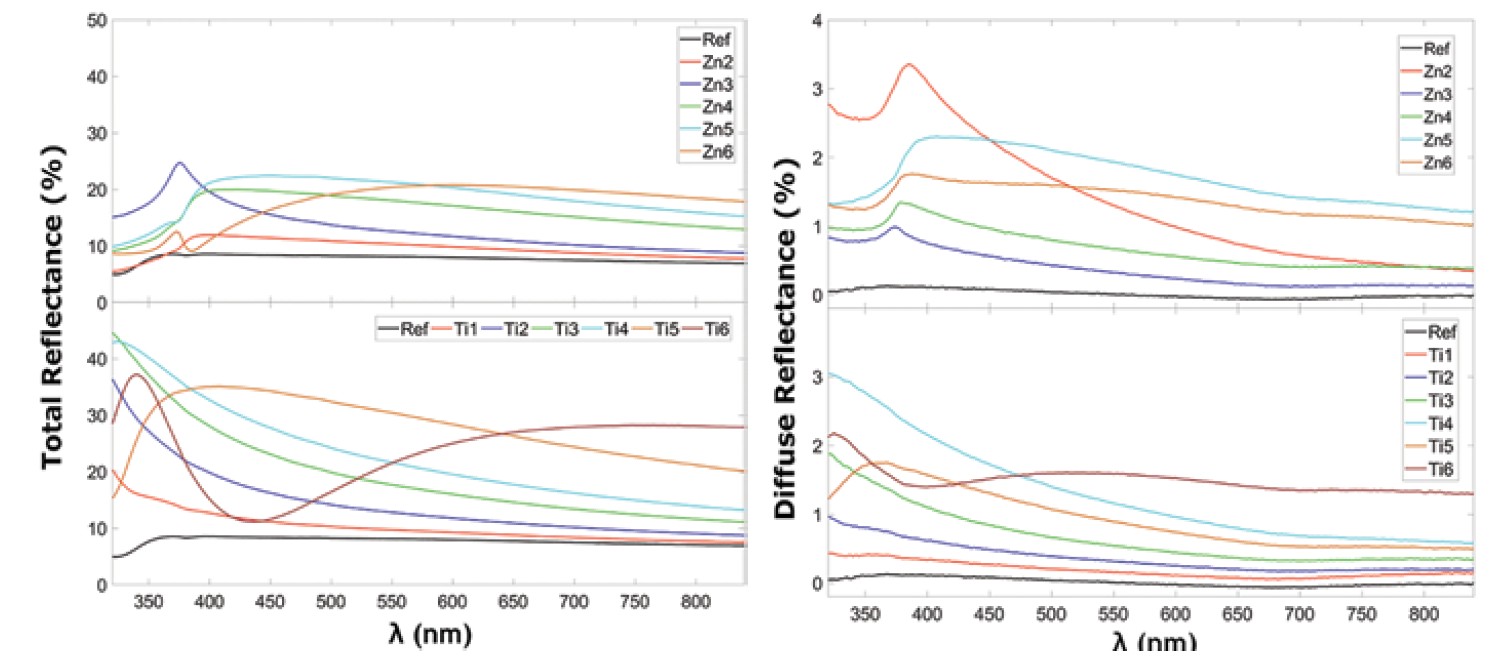
Figure 1. (a) Total reflectance and (b) diffuse reflectance of thin film-coated glass samples. Credit: Johansson et al., Frontiers in Materials (CC BY 4.0)
The coated glass showed a potential improvement in life expectancy of PV modules through a decrease of destructive UV-radiation transmission to the encapsulant up to a relative 36.0% and 54.3% for TiO2 and ZnO coatings, respectively. Additionally, although the coated samples have shown a relative transmission reduction at the useful spectral region up to 21.8 and 12.3% for TiO2 and ZnO coatings, respectively, the transmissivity degradation of the encapsulant should be effectively prevented.
For ZnO it is evident that the Fe3+ content plays an important role for the UV-blocking activity, which would be a tradeoff between limiting the glass’s iron content while still having enough UV protection. Furthermore, ZnO-coated glass also showed potential regarding down conversion of UV light to visible wavelength with peaks at 377 nm and 640 nm. Thus, ZnO is feasible to be investigated for application as coating to cover glasses of PV modules but must be optimized as there is a tradeoff between UV-blocking and transmittance in the useful spectral region for PV modules (Table 3).
Implications for PV modules
We have shown that UV blocking can be achieved with the cost of reducing the transmittance. This opens the possibility for maintaining UV protection and gaining useful energy for the PV by lowering the Fe2O3 content in the glass without compromising the service lifetime of the PV module. The energy balance for transmitted and useful light for PV modules will be possible to model and optimize in future studies based on information as, for instance, possible limits for Fe2O3 content, cost, and efficiency.
Furthermore, photon energy down-conversion, i.e., photoluminescence, can be an advantage and a route to utilizing UV light while still not exposing the PV cells to UV light. As for disadvantages, we can list higher reflectivity and scattering. If the surface coating is properly structured, it might not be a serious disadvantage or perhaps even an advantage,19 as the diffused light contains in fact more photons than the direct light of normal incidence. This is especially valid for façade-applied PV modules where there is in fact very little solar radiation of normal incidence.
Another parameter not previously mentioned is the factor of heat. A photon’s energy that is not converted into electricity is transformed into heat that in fact lowers the efficiency of the PV module. Beyond the scope of the current paper we would also like to draw the attention to making crystalline ZnO or TiO2 coatings having similar beneficial properties but with the added value of photocatalysis20,21 and hydrophilic behavior with UV exposure,22,23 thereby giving PV-covered glasses reduced maintenance. Doped ZnO also offers another dimension as a transparent conductive coating offering possible IR reflection for wavelengths nonconvertible to energy for PV modules.8
Acknowledgments
Jakob Thyr at the Ångström Laboratory, Uppsala University is greatly acknowledged for his supervision during the photoluminescence measurements.
This article is excerpted from “Transparent TiO2 and ZnO thin films on glass for UV protection of PV modules,” Frontiers in Materials, October 2019 (CC BY 4.0). https://doi.org/10.3389/fmats.2019.00259
Cite this article
W. Johansson, A. Peralta, B. Jonson, S. Anand, L. Österlund, and S. Karlsson, “Transparent TiO2 and ZnO thin films on glass for UV protection of PV modules,” Am. Ceram. Soc. Bull. 2020, 99(4): 26–29.
About the Author(s)
Wilhelm Johansson and Bo Jonson are student and professor, respectively, in the Department of Built Environment and Energy Technology at Linnaeus University (Sweden). Albert Peralta and Srinivasan Anand are student and professor, respectively, in the Department of Applied Physics at the KTH Royal Institute of Technology (Sweden). Lars Österlund is professor in the Department of Engineering Sciences at Uppsala University (Sweden). Stefan Karlsson is senior scientist and project manager at RISE Research Institutes of Sweden, Glass section (Sweden). Contact Karlsson at stefan.karlsson@ri.se.
Issue
Category
- Energy materials and systems
- Glass and optical materials
Article References
1IPCC (2014). “Summary for policymakers,” in Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds O. Edenhofer et al. (Cambridge, UK; New York, NY: Cambridge University Press).
2IEA (2011). Solar Energy Perspectives. Paris: International Energy Agency.
3Masson, G., Kaizuka, I., and Cambiè, C. (2018). IEA Report PVPS: A Snapshot of Global PV (1992-2017). International Energy Agency.
4Czanderna, A. W., and Pern, F. J. (1996). “Encapsulation of PV modules using ethylene vinyl acetate copolymer as a pottant: a critical review,” Solar Energy Mater. Solar Cells 43, 101–181. doi: 10.1016/0927-0248(95)00150-6
5Oliveira, M. C. C. D., Diniz Cardoso, A. S. A., Viana, M. M., and Lins, V. d.F.C. (2018). “The causes and effects of degradation of encapsulant ethylene vinyl acetate copolymer (EVA) in crystalline silicon photovoltaic modules: A review,” Renew. Sust. Energy Rev. 81, 2299–2317. doi: 10.1016/j.rser.2017.06.039
6Burrows, K., and Fthenakis, V. (2015). “Glass needs for a growing photovoltaics industry,” Solar Energy Mater. Solar Cells 132, 455–459. doi: 10.1016/j.solmat.2014.09.028
7Brow, R. K., and Schmitt, M. L. (2009). “A survey of energy and environmental applications of glass,” J. Eur. Ceram. Soc. 29, 1193–1201. doi: 10.1016/j.jeurceramsoc.2008.08.011
8Deubener, J., Helsch, G., Moiseev, A., and Bornhöft, H. (2009). “Glasses for solar energy conversion systems,” J. Eur. Ceram. Soc. 29, 1203–1210. doi: 10.1016/j.jeurceramsoc.2008.08.009
9Nielsen, K. H., Orzol, D. K., Koynov, S., Carney, S., Hultstein, E., and Wondraczek, L. (2014). “Large area, low cost anti-reflective coating for solar glasses,” Solar Energy Mater. Solar Cells 128, 283–288. doi: 10.1016/j.solmat.2014.05.034
10Bamford, C. R. (1982). “Optical properties of flat glass,” J. Non Cryst. Solids 47, 1–20. doi: 10.1016/0022-3093(82)90342-8
11Rubin, M. (1985). “Optical properties of soda lime silica glasses,” Solar Energy Mater. 12, 275–288. doi: 10.1016/0165-1633(85)90052-8
12Volotinen, T. T., Parker, J. M., and Bingham, P. A. (2008). “Concentrations and site partitioning of Fe2+ and Fe3+ ions in a soda-lime-silica glass obtained by optical absorbance spectroscopy,” Phys. Chem. Glasses Euro. J. Glass Sci. Technol. B 49, 258–270. Available online at: https://www.ingentaconnect.com/content/sgt/ejgst/2008/00000049/00000005/art00004
13Daneo, A. G., Falcone, R., and Hreglich, S. (2009). “Effect of the redox state on container glass colour stability,” Glass Technol. Euro. J. Glass Sci. Technol. A 50, 147–150. Available online at: https://www.ingentaconnect.com/content/sgt/gta/2009/00000050/00000003/art00004
14Goodyear, J. K., and Lindberg, V. L. (1980). “Low absorption float glass for back surface solar reflectors,” Solar Energy Mater. 3, 57–67. doi: 10.1016/0165-1633(80)90049-0
15Allsopp, B. L., Christopoulou, G., Brookfield, A., Forder, S. D., and Bingham, P. A. (2018). “Optical and structural properties of d0 ion-doped silicate glasses for photovoltaic applications,” Phys. Chem. Glasses Euro. J. Glass Sci. Technol. B 59, 193–202. doi: 10.13036/17533562.59.4.003
16Osterwald, C. R., Benner, J. P., Pruett, J., Anderberg, A., Rummel, S., and Ottoson, L. (2003). “Degradation in weathered crystalline-silicon PV modules apparently caused by UV radiation,” in 3rd World Conference on Photovoltaic Energy Conversion (Osaka).
17Kuitche, J. M., Pan, R., and TamizhMani, G. (2014). “Investigation of dominant failure mode(s) for field-aged crystalline silicon PV modules under desert climatic conditions,” IEEE J. Photovoltaics 4, 814–826. doi: 10.1109/JPHOTOV.2014.2308720
18Jordan, D. C., and Kurtz, S. R. (2013). “Photovoltaic degradation rates—an analytical review,” Prog. Photovoltaics Res. Appl. 21, 12–29. doi: 10.1002/pip.1182
19Brongersma, M. L., Cui, Y., and Fan, S. (2014). “Light management for photovoltaics using high-index nanostructures,” Nat. Mater. 13, 451–460. doi: 10.1038/nmat3921
20Gao, Y., and Nagai, M. (2006). “Morphology evolution of ZnO thin films from aqueous solutions and their application to solar cells,” Langmuir 22, 3936–3940. doi: 10.1021/la053042f
21He, H., Liu, C., Dubois, K. D., Jin, T., Louis, M. E., and Li, G. (2012). “Enhanced charge separation in nanostructured TiO2 materials for photocatalytic and photovoltaic applications,” Ind. Eng. Chem. Res. 51, 11841–11849. doi: 10.1021/ie300510n
22Watanabe, T., Nakajima, A., Wang, R., Minabe, M., Koizumi, S., Fujishima, A., et al. (1999). “Photocatalytic activity and photoinduced hydrophilicity of titanium dioxide coated glass,” Thin Solid Films 351, 260–263. doi: 10.1016/S0040-6090(99)00205-9
23Sun, R.-D., Nakajima, A., Fujishima, A., Watanabe, T., and Hashimoto, K. (2001). “Photoinduced surface wettability conversion of ZnO and TiO2 thin films,” J. Phys. Chem. B 105, 1984–1990. doi: 10.1021/jp002525j
Related Articles
Market Insights
Engineered ceramics support the past, present, and future of aerospace ambitions
Engineered ceramics play key roles in aerospace applications, from structural components to protective coatings that can withstand the high-temperature, reactive environments. Perhaps the earliest success of ceramics in aerospace applications was the use of yttria-stabilized zirconia (YSZ) as thermal barrier coatings (TBCs) on nickel-based superalloys for turbine engine applications. These…
Market Insights
Aerospace ceramics: Global markets to 2029
The global market for aerospace ceramics was valued at $5.3 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 8.0% to reach $8.2 billion by the end of 2029. According to the International Energy Agency, the aviation industry was responsible for 2.5% of…
Market Insights
Innovations in access and technology secure clean water around the world
Food, water, and shelter—the basic necessities of life—are scarce for millions of people around the world. Yet even when these resources are technically obtainable, they may not be available in a format that supports healthy living. Approximately 115 million people worldwide depend on untreated surface water for their daily needs,…




