Ceramic materials offer many desirable properties: high thermal and chemical stability, strong mechanical properties, and biocompatibility, among others.
Yet these properties can make it difficult to shape and sinter ceramics, especially components featuring complex geometries, such as hierarchical patterns and internal structures.
Additive manufacturing (AM) is seen as a transformative method to fabricate geometrically complex ceramic components through precise layer-by-layer deposition. But while research in ceramic AM has demonstrated significant potential to save time, materials, and energy, challenges in using these techniques remain.
For example, most ceramic AM processes still require long (up to several days) post-shaping thermal treatments at temperatures above 1,000°C to remove organic additives (debinding) and densify the ceramic part (sintering).1 These treatments limit the rapid production of ceramic parts and, when conducted in a furnace, do not allow a tunable energy delivery for each layer. These treatments also pose significant challenges for multimaterial fabrication, particularly during co-sintering. When materials with differing thermal expansion rates are processed together, mismatched shrinkage kinetics can lead to defects such as distortion, cracking, or delamination. These issues are especially common when co-sintering dissimilar materials, such as ceramics, metals, and glasses.
To mitigate such risks, it is generally necessary to use materials that sinter under similar thermal and atmospheric conditions. As a result, material selection for multimaterial components is highly restricted, limiting the design of advanced functionalities.
These constraints could be alleviated through layer-specific heating, enabling the integration of materials with varying thermal expansion and shrinkage behaviors into complex, multimaterial parts. This approach may also eliminate the need for prolonged high-temperature, post-processing treatments, which offers clear technological, economic, and environmental benefits.
Alternative heat sources and low-temperature chemical methods are both possible approaches to layer-specific heating of additively manufactured ceramics. Recent advancements in innovative sintering technologies—such as leveraging electric fields, rapid heating rates, pressure, or hydrothermal conditions—show great promise for integration with AM to produce high-performance ceramics efficiently.1
Amongst alternative heating routes, high-energy light irradiation—also called photonic curing or photonic sintering—has shown promise in densifying ceramic thin films (<1 µm thick)2–6 and, more recently, thicker layers (tens of microns)7–10 over sufficiently short timescales for use in AM.
Photonic curing uses high-intensity, short-duration light pulses (usually in the millisecond range) to selectively heat a material’s surface or deposited layer without significantly heating the underlying substrate. Current technologies allow precise control over the shape, frequency, and other features of the emitted pulses, giving a lot of freedom to the photonic treatment for annealing thin films, sintering metal lines on temperature-sensitive substrates, reflowing solder, activating dopants, and improving the crystallinity of semiconductors, among other applications.
However, photonic curing faces the challenges of limited penetration depth, nonuniform sintering if energy absorption is not optimized, and the need for compatible inks and materials that absorb light efficiently, among other hindrances.
With funding from the U.S. Office of Naval Research, Mark Losego’s group at Georgia Institute of Technology runs a project called “Exploration of new chemistries and processes for additive manufacturing of ceramics” (grant no. N00014-21-1-2258). Through this project, we aim to develop a new, single-step ceramic additive manufacturing process that does not require post-process pyrolysis or high-power lasers. To achieve this goal, we are developing new ceramic and preceramic chemistries that can achieve proper densification through liquid-phase sintering at low temperatures (<1,000°C).
A key point of this development process is the use of an inorganic binder that experiences consolidation reactions at temperatures low enough to minimize unwanted transformations of the main material. Aluminum dihydrogen phosphate (Al(H2PO4)3, ADP) is a particularly attractive binder option due to the feasibility of creating chemically bonded phosphate ceramics (CBPCs)11–13 by combining a layer-by-layer deposition process with light irradiation to trigger the binder consolidation and phosphate bonding with ceramic particles.14–16
CBPCs are composites that are obtained by dissolving an inorganic phosphate binder along with a dense ceramic powder in a solvent and subsequently reassembling the dissolved components into a new solid structure. This acid–base-type CBPC synthesis reaction usually takes place between an anion donor phosphate binder—such as phosphoric acid (H3PO4), diammonium hydrogen phosphate ((NH4)2HPO4), or ADP—and a metal oxide, such as magnesium oxide or alumina. These systems undergo inorganic condensation polymerization reactions that form ceramic phosphate phases bound together by PO4 tetrahedral linkages (Figure 1).
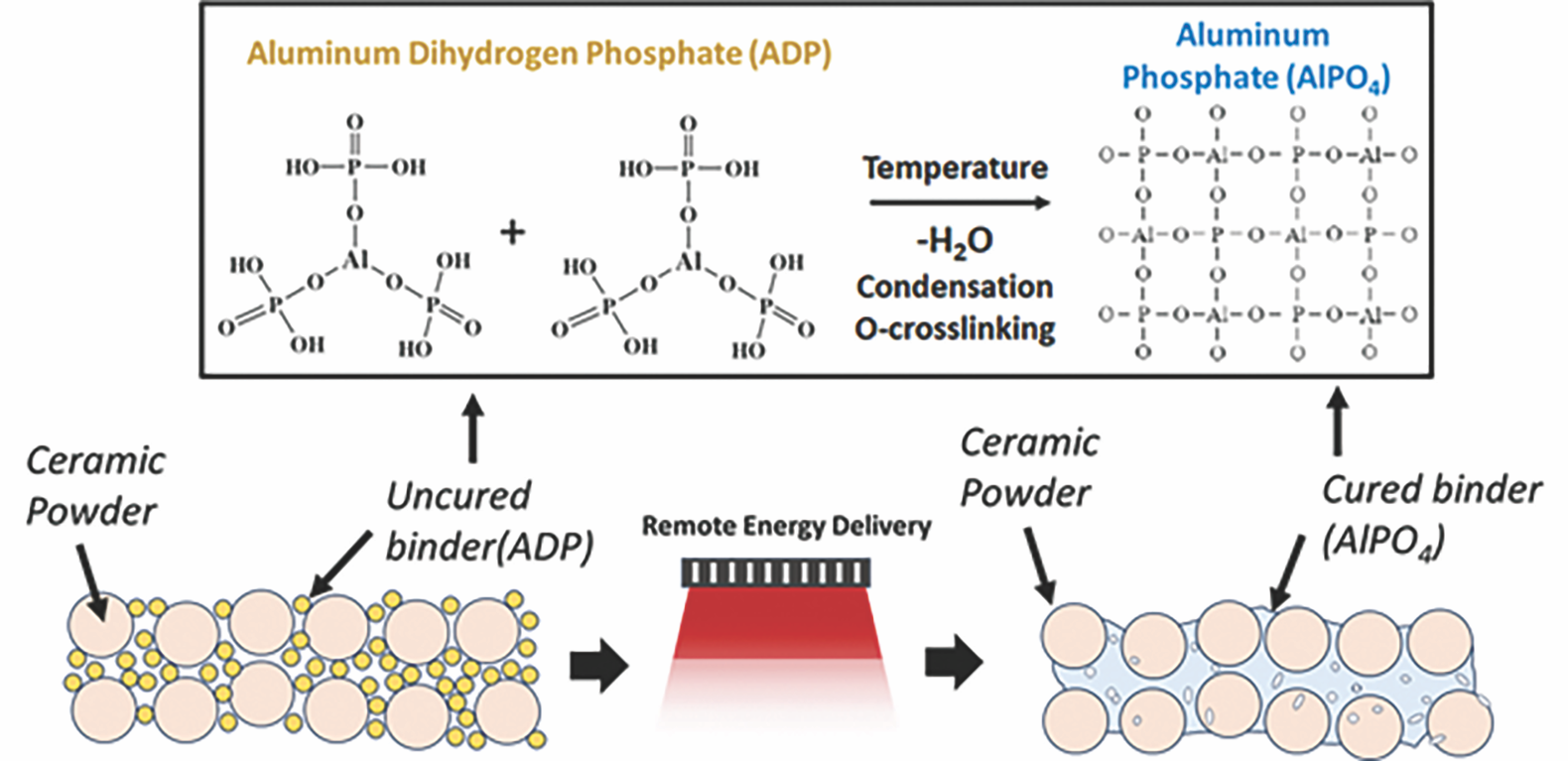
Figure 1. Overview of the condensation and crosslinking mechanism of aluminum dihydrogen phosphate as a binder. Its binding effect with ceramic particles is based on adhesion processes and on the same chemical phosphate crosslinks. Credit: Nicolas Somers
The formation of CBPCs occurs at low temperatures (<500°C). Thus, CBPCs have lower energy requirements than sintered ceramics while retaining high chemical resistance, high compressive strength, high abrasion resistance, biocompatibility, and good dimensional and thermal stability, often above 1,000°C. Additionally, the versatile chemistry of CBPCs allows functionality to be added to the final product, for example, corrosion resistance by doping with molybdate or chromate, self-healing capability by doping with cerium oxide, semiconductivity by doping with indium and its oxides, and thermochromism by doping with vanadium or chromium oxides.
Transposing this concept to an AM configuration represents a new route for ceramic AM, with opportunities for new functionalities and applications of multimaterial 3D structures. The new approach would remove the need for post-shaping thermal treatments thanks to the triggering of the reactive phosphate binder through infrared or visible light irradiation. The tailored heating of each layer along with the use of an efficient binder should offer new opportunities to combine materials with different thermal expansion coefficients and shrinkage kinetics into complex, multimaterial parts.
So far, our group has published four articles regarding the association of inorganic phosphate binders, photonic curing, and additive manufacturing.
Exploration of additively manufactured CBPCs
First article: Developing the infrared-driven curing process
Our first article introduced a fast, low-temperature, pressureless process to chemically bind ceramic parts with the help of infrared (IR) irradiation and phosphate binder condensation (Figure 2).16
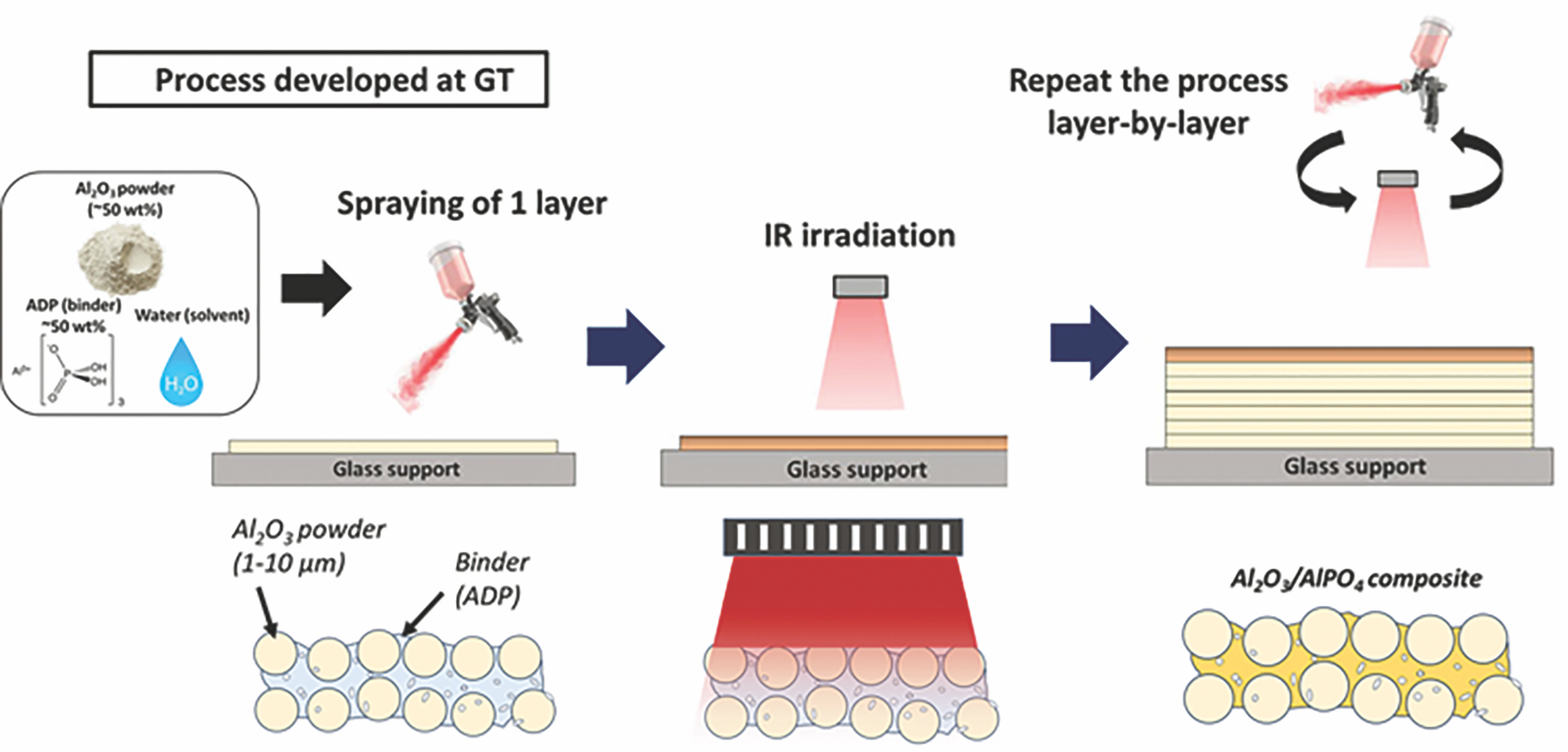
Figure 2. Schematic of the new synthesis process, which involves depositing a ceramic slurry through a high-volume, low-pressure spray gun followed by infrared curing of the sprayed layers. Adapted from Reference 16. Credit: Somers et al., JACerS
Ceramic components are synthesized from slurries of ceramic powders and ADP binders that are spray cast and irradiated with short-waved IR light capable of heating the system to 350°C. This irradiation is found to be sufficient to drive phosphate condensation, binding the ceramic powders together within a matter of seconds. The IR-irradiated components show an increase in density and Vickers hardness relative to uncured samples.
This approach allowed the production of free-standing ceramic sheets consisting of different oxide ceramics, such as alumina, iron oxide, silica, indium oxide, copper oxide, and tin oxide (Figure 3). These freestanding ceramic sheets were sufficiently robust to be handled, and they presented an important interconnected porosity that could find interesting applications in refractories, bone implants, electronics, and thermal barrier coatings, among other fields.
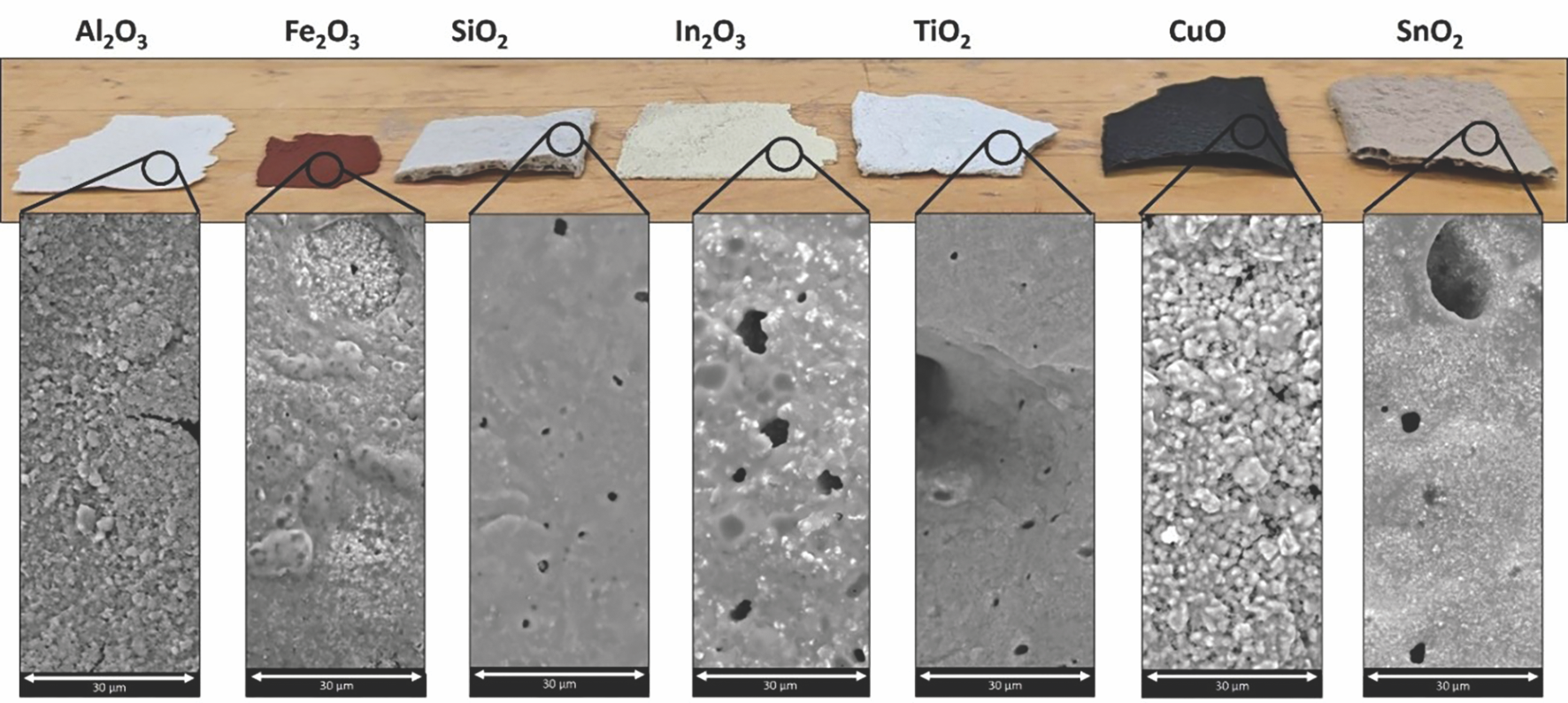
Figure 3. Photographs (top) and micrographs (bottom) of 20-layer, spray-cast, and infrared-irradiated ADP-bound ceramic sheets created from various oxide powders.16 Credit: Somers et al., JACerS
Second article: Optimization of printed parts
To optimize the properties of the printed parts, our second article investigated using hydrothermal treatments to modify the ceramic powder’s surface chemistry to make it more reactive to the phosphate binder phase and, ideally, enhance mechanical strength and potentially density.15 Specifically, we explored whether hydrothermal treatments could increase the number of hydroxyl and phosphate groups on the alumina powders (Figure 4a).
Alumina powders were pretreated in hydrothermal conditions with both water and phosphoric acid solution before slurry preparation and infrared irradiation. The effects of hydrothermal treatments on the powder’s chemical reactions and the composite’s final microstructure were assessed upon IR irradiation.
While water treatment did not induce any chemical changes, the presence of phosphoric acid led to the appearance of phosphate phases Al(PO3)3 and AlPO4. Hydrothermal treatments in water and phosphoric acid solution were found to drive faster and more intense phosphate condensation reactions at lower temperatures, with a stronger effect in the presence of H3PO4 (condensation temperature of 150°C for the H3PO4-hydrothermal treated powder compared to 165°C for the untreated powder).
This improved reactivity of hydrothermally treated α-Al2O3 powders, especially in the presence of H3PO4, leads to a general decrease of porosity for 3D-printed parts compared to untreated alumina (Figure 4b). While further optimization is needed to reduce the final porosity, it is possible to rapidly print 3D parts using this method, demonstrating a possible pathway to a single-step ceramic AM process.
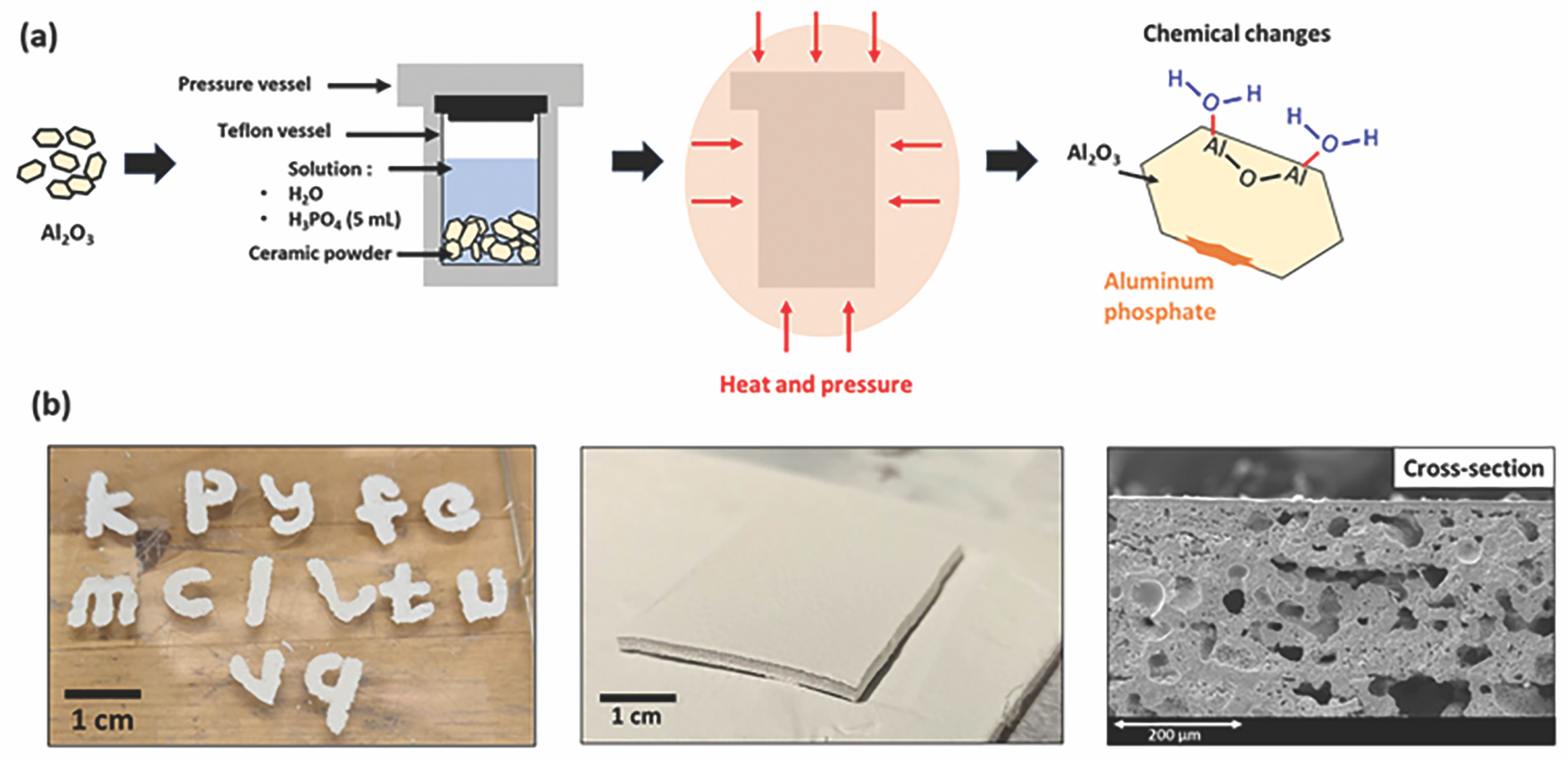
Figure 4. (a) Schematic of the hydrothermal treatment of alumina, which induced chemical changes at the surface of the particles. (b) Photographs and scanning electron microscopy micrographs of the H3PO4-treated powder that was shaped with spraying and infrared irradiation. Credit: Nicolas Somers
Third article: Creation of thermochromic sensor
Our third article involved manufacturing an inorganic, high-temperature (~600°C) thermochromic sensor made of a CBPC.14 It showed the feasibility of fabricating CBPCs with reversible thermochromic properties using photonic annealing methods that follow a process flow consistent with additive manufacturing.
Phase analysis confirmed that the remote photonic energy can transform the ADP binder into an AlPO4 ceramic phase that binds the thermochromic powders together. Interestingly, the quantity of energy needed to achieve “full conversion” of ADP to AlPO4 is approximately the same for both IR irradiation and flash-lamp annealing (FLA), about 300 J/cm2. However, FLA can deliver this energy in nearly two orders of magnitude less time due to being in the visible light spectrum, making it even more amenable for rapid prototyping.
The FLA system (PulseForge Invent) enabled easy adjustment of the photonic pulse sequence. We found that an initial sequence of low-energy pulses (10 flashes with 100 V or 1.1 J/cm2 of lamp power, for a total energy of 11 J/cm2) was important to first remove physically absorbed water prior to driving ceramization. This “drying step” was followed by higher energy pulses at a range of lamp voltages varying from 400 to 520 V, each with 30 repetitions, providing energy densities of 207, 279, 327, and 399 J/cm2, respectively, paralleling those provided by the IR lamp. Because energy delivery was varied by lamp voltage, the duration of each FLA process could be kept constant at 48 seconds.
By understanding the thermochromic color changes of Cr:Al2O3 under varying conditions, we manufactured a simple, patterned thermochromic device to demonstrate the potential for this technology (Figure 5). Future work will need to optimize similar slurries for integration with higher resolution printing methods, such as inkjet printing and aerosol jet printing. Additionally, stacking of multiple layers and understanding their interaction, both internally and in conjunction with the FLA method, will bring a new approach to rapid additive manufacturing.
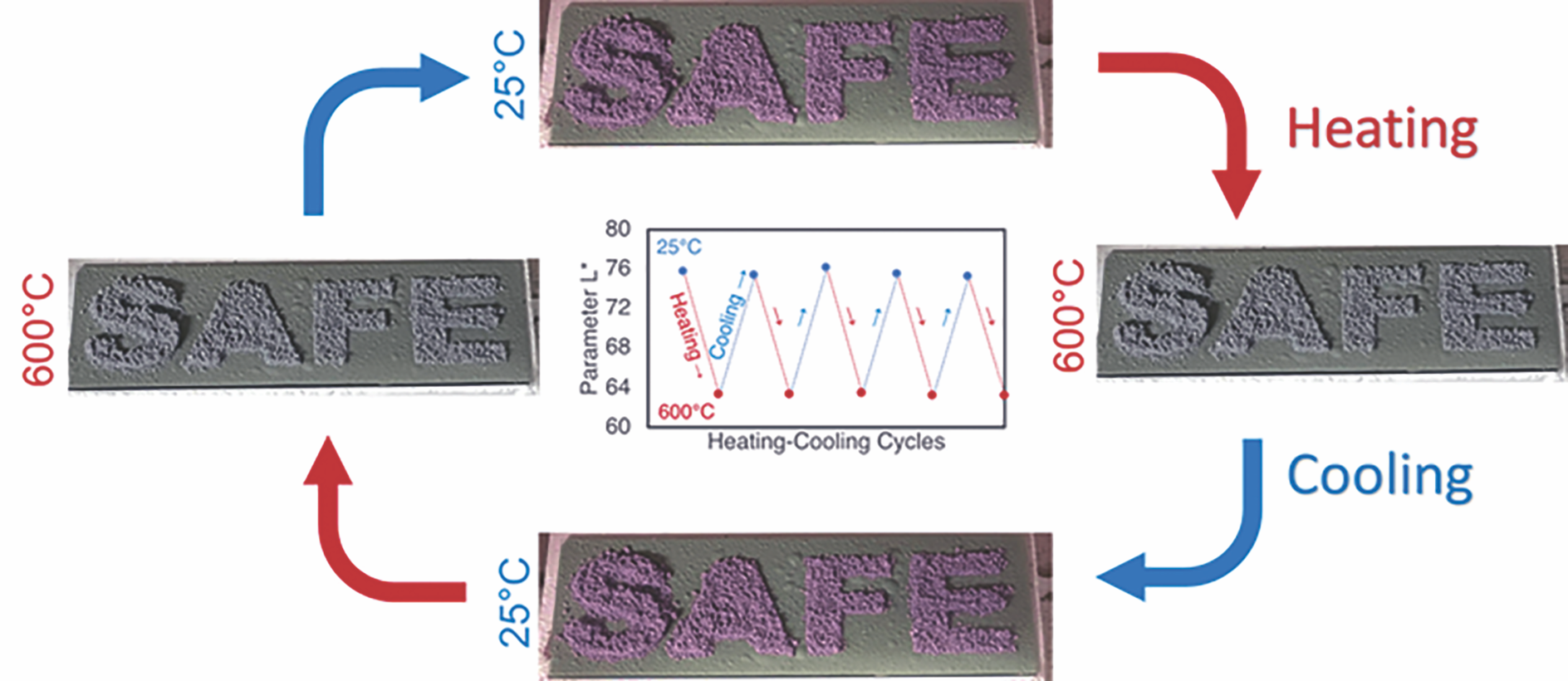
Figure 5. Glass slides prepared with letters of 10 wt.% Cr:Al2O3–ADP (solid-state synthesis at 1,400°C) and surrounding media of 10 wt.% Cr:Al2O3–ADP (solid-state synthesis at 1,000°C). Color change of letters at 600°C makes them disappear within the background, warning users that it is no longer safe to touch. Adapted from Reference 14. Credit: Özmen et al., Adv. Eng. Mater.(CC BY-NC-ND 4.0)
Fourth article: Exploring the influence of binder:powder ratio
In our fourth article, we examined the influence of the ADP (binder):ceramic powder ratio and concentration, as well as the chemistry of optical absorber additives, on the overall density and microstructure of CBPC composite coatings composed of AlPO4 and Al2O3.17 Our aim was to optimize the chemical composition, especially the ADP to alumina ratio, and the absorbance of the ceramic layers at wavelengths of the xenon lamp (400–800 nm), which significantly affect the bonding mechanisms, microstructure, and mechanical properties of the final ceramic parts.
This work showed that the ADP (binder):ceramic powder ratio affects the microstructure, porosity, and conversion efficiency in CBPC composites. The optimal ratio appears to be near 55 vol.% ADP to achieve layers that do not crack and convert all the ADP to the AlPO4 phase (Figure 6). It is possible to convert 100-µm-thick, blade-cast layers into CBPC composites with an FLA pulse within 20 seconds. However, the photothermal conversion efficiency can be further enhanced by adding optical light absorbers to these CBPC slurries, lowering the necessary photonic energy density by more than 30%, from 300 to 220 J/cm2 (Figure 7).
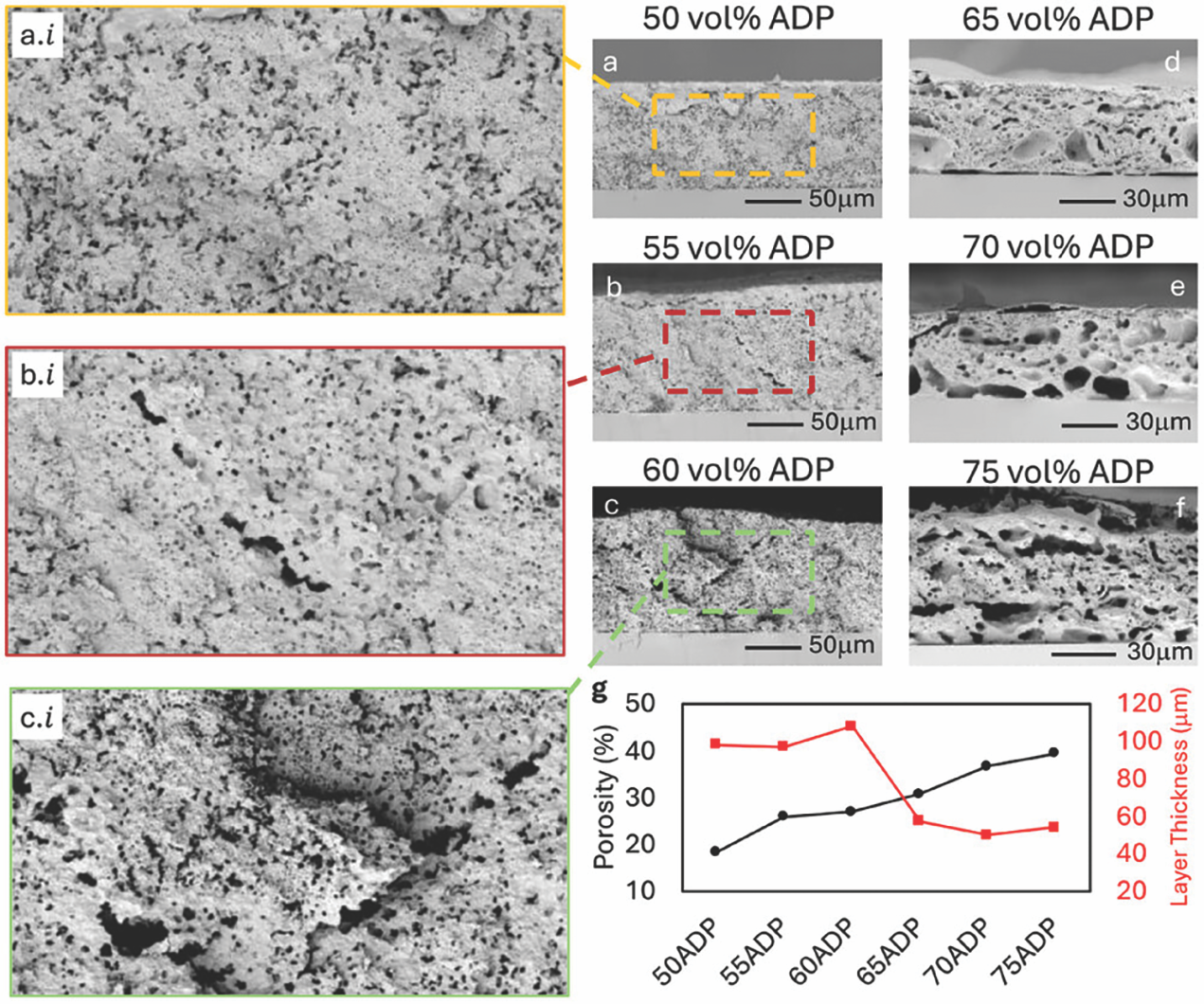
Figure 6. Cross-sectional scanning electron microscopy images of ADP–Al2O3 layers cured in a furnace at 400°C for two hours with ADP vol.% of (a) 50%, (b) 55%, (c) 60%, (d) 65%, (e) 70%, and (f) 75%. (g) Plotted change in porosity and cured layer thickness versus increasing ADP vol.%.17 Credit: Özmen et al., IJCES(CC BY 4.0)
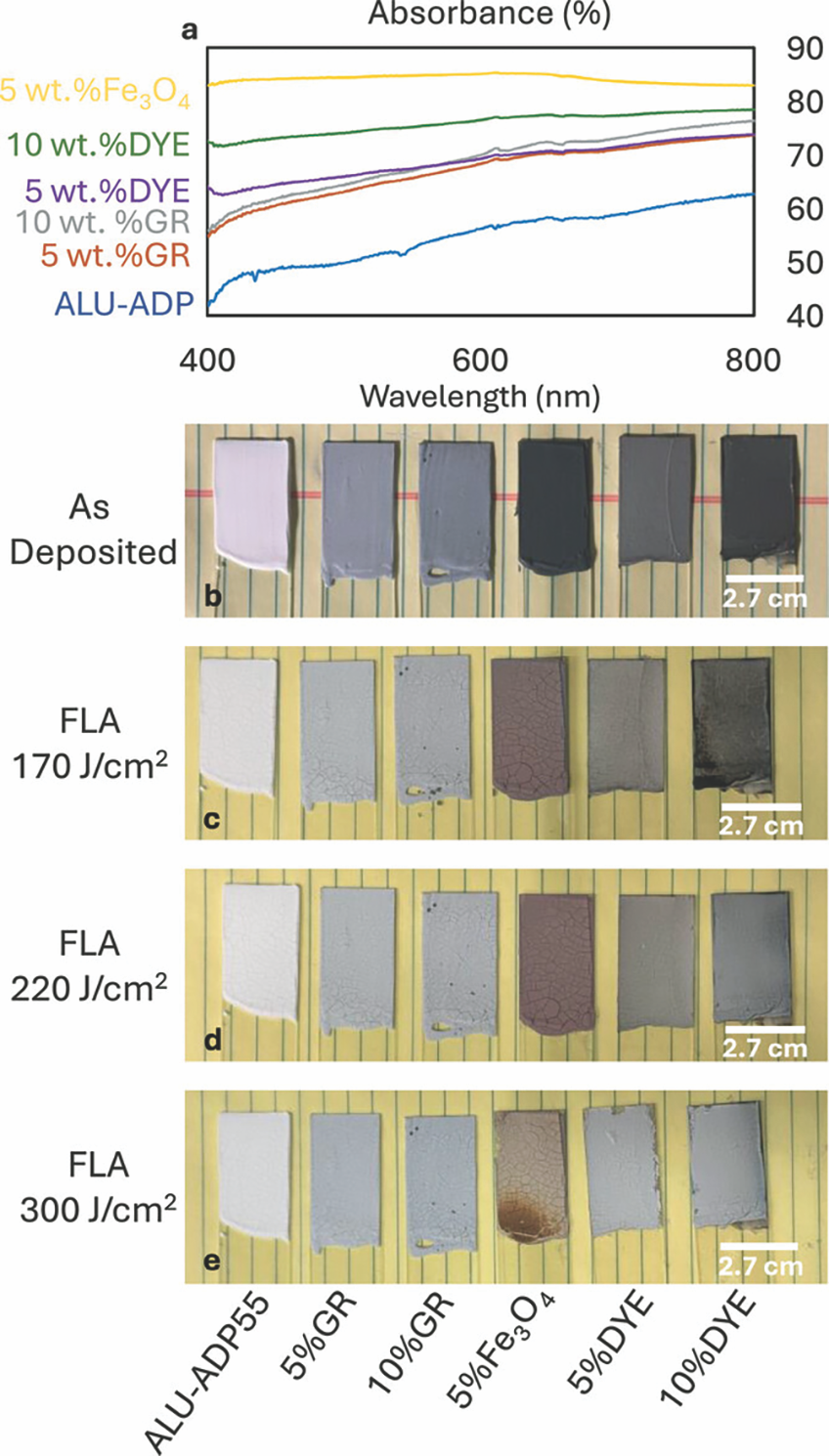
Figure 7. (a) Absorbance spectra for as-cast ADP–Al2O3 layers without any optical absorbers (blue) and with 5 wt.% graphite (orange), 10 wt.% graphite (gray), 5 wt.% black iron oxide (yellow), 5 wt.% black liquid dye (purple), and 10 wt.% black liquid dye (green). (b–e) Photos of these same ADP55–Al2O3 layers without any optical absorber and with 5 and 10 wt.% graphite, 5 wt.% black iron oxide, and 5 and 10 wt.% dye.17 Credit: Özmen et al., IJCES (CC BY 4.0)
Of the light absorber additives tested here, a molecular liquid black dye was most interesting because it easily mixed with the slurry and provided good photothermal energy conversion. Future opportunities exist to further engineer this system for functional coatings or even extend to layer-by-layer printing for additive manufacturing. Perspectives for biomedical applications Over the last two decades, the promise of patient-specific synthetic grafts for bone repair has been a strong driving force in the development of ceramic AM. Several bioceramics and bioactive glasses have been identified as suitable synthetic graft materials due to their biocompatibility, bioactivity and bioresorbability,18 and efforts are underway to shape these materials into complex architectures that interact with host cells to trigger a regenerative response, osteogenesis, and vascularization.19,20
However, translating the encouraging outcomes from academic research into the clinic remains a challenge: Worldwide, only three companies are currently commercializing ceramic 3D-printed implants.21 As in traditional ceramic manufacturing, most ceramic AM processes still require long post-shaping thermal treatments at temperatures above 1,000°C. These treatments alter the physicochemical characteristics of the initial bioceramics and, therefore, their bioactivity and resorbability.
For example, bioresorption is usually faster for amorphous biomaterials than for crystallized ones. Ideally, the rate of bioresorbability of the implant should match the rate of tissue regeneration while keeping sufficient mechanical strength. Producing such gradually and fully resorbable bioceramic scaffolds with AM technologies is a challenge because the usual sintering treatments lead to high crystallinity of materials.
The tailored heating of each layer allowed by photonic curing could overcome these challenges with processing additively manufactured bioceramics and bioactive glasses.
The future of AM processing
Conventional sintering of additively manufactured multi-material components can lead to structural defects such as distortion, cracks, and delaminations unless the selected materials have matching properties and can be sintered in the same temperature range and atmosphere. The new photonic curing approach developed at Georgia Tech could overcome these challenges, opening the door to the adoption of multimaterial AM in a broad range of potential applications in the biomedical field and beyond.
Acknowledgments
This research was supported by a grant from the U.S. Office of Naval Research (grant no. N00014-21-1-2258) under the direction of Antti Makinen. A portion of this work was also conducted in the Materials Innovation and Learning Laboratory, an open-access materials characterization facility supported by the School of Materials Science and Engineering and the College of Engineering at Georgia Tech. The flash lamp annealing equipment used in this study was purchased with an ONR-DURIP grant (grant no. N00014-23-1-2076).
Cite this article
N. Somers, E. Özmen, and M. D. Losego, “Additive manufacturing of porous ceramics: Direct processing through phosphate condensation and photonic irradiation,” Am. Ceram. Soc. Bull. 2025, 104(7): 30–34.
About the Author(s)
Nicolas Somers is postdoctoral researcher at the University of Liège, Belgium. Eren Özmen is postdoctoral researcher and Mark Losego is professor at Georgia Institute of Technology. Contact Losego at losego@gatech.edu.
Issue
Category
- Manufacturing
Article References
1A. Zocca et al., “Additive manufacturing of ceramics: Issues, potentialities, and opportunities,” Journal of the American Ceramic Society 2015, 98(7): 1983–2001.
2K. Mallikarjuna et al., “Photonic drying/annealing: Effect of oven/visible light/infrared light/flash-lamp drying/annealing on WO3 for electrochromic smart windows,” ACS Sustain Chem Eng 2021, 9(43): 14559–14568.
3M. Hösel, and F. C. Krebs, “Large-scale roll-to-roll photonic sintering of flexo printed silver nanoparticle electrodes,” J. Mater. Chem. 2012, 22(31): 15683–15688.
4M. Trzaskowska, V. Vivcharenko, and A. Przekora, “The impact of hydroxyapatite sintering temperature on its microstructural, mechanical, and biological properties,” International Journal of Molecular Sciences 2023, 24(6): 5083.
5Y. H. Son et al., “Application of flash-light sintering method to flexible inkjet printing using anti-oxidant copper nanoparticles,” Thin Solid Films 2018, 656: 61–67.
6E. B. Secor et al., “Rapid and versatile photonic annealing of graphene inks for flexible printed electronics,” Advanced Materials 2015, 27(42): 6683–6688.
7E. Gilshtein et al., “Millisecond photonic sintering of iron oxide doped alumina ceramic coatings,” Scientific Reports 2021, 11: 3536.
8L. Porz et al., “Blacklight sintering of ceramics,” Mater. Horiz. 2022, 9(6): 1717–1726.
9M. Scherer et al., “Blacklight sintering of BaTiO3 ceramics,” J. Eur. Ceram. Soc. 2023, 43(12): 5406–5411.
10E. Gilshtein et al., “Photonic sintering of oxide ceramic films: Effect of colored FexOy nanoparticle pigments,” Ceramics 2022, 5(3): 351–361.
11A. S. Wagh, “Recent progress in chemically bonded phosphate ceramics,” ISRN Ceramics 2013, 2013: 983731.
12A. S. Wagh, “Chapter 2: Chemically bonded phosphate ceramics,” in Chemically Bonded Phosphate Ceramics (2nd edition). Elsevier, 2016. pp. 17–34.
13S. Y. Jeong, and A. S. Wagh, “Cementing the gap between ceramics, cements, and polymers,” Materials Technology 2003, 18(3): 162–168.
14E. Özmen, N. Somers, and M. D. Losego, “Rapid, direct fabrication of thermochromic ceramic composite sensors via flash lamp annealing,” Adv. Eng. Mater. 2024, 26(11): 2400323.
15N. Somers, E. Özmen, and M. D. Losego, “Hydrothermal treatment of alumina powders to alter the low-temperature binding of chemically bonded phosphate ceramic composites via infrared irradiation,” J. Mater. Sci. 2024, 59: 4089–4101.
16N. Somers et al., “Infrared irradiation to drive phosphate condensation as a route to direct additive manufacturing of oxide ceramics,” Journal of the American Ceramic Society 2023, 107(1): 36–46.
17E. Özmen and M. D. Losego, “Effects of the concentration of inorganic binders and optical absorbers on the phase formation and microstructure of flash-lamp-annealed chemically bonded phosphate ceramic composites,” International Journal of Ceramic Engineering & Science 2025, 7(4): e70019.
18H. Qu et al., “Biomaterials for bone tissue engineering scaffolds: A review,” RSC Adv 2019, 9(45): 26252–26262.
19D. Han, and H. Lee, “Recent advances in multi-material additive manufacturing: methods and applications,” Current Opinion in Chemical Engineering 2020, 28: 158–166.
20T. Moritz and S. Maleksaeedi, “Chapter 4: Additive manufacturing of ceramic components,” in Additive Manufacturing: Materials, Processes, Quantifications and Applications. Elsevier, 2018. pp. 105–161.
21P. Chocholata, V. Kulda, and V. Babuska, “Fabrication of scaffolds for bone-tissue regeneration,” Materials 2019, 12(4): 568.
Related Articles
Market Insights
Engineered ceramics support the past, present, and future of aerospace ambitions
Engineered ceramics play key roles in aerospace applications, from structural components to protective coatings that can withstand the high-temperature, reactive environments. Perhaps the earliest success of ceramics in aerospace applications was the use of yttria-stabilized zirconia (YSZ) as thermal barrier coatings (TBCs) on nickel-based superalloys for turbine engine applications. These…
Market Insights
Aerospace ceramics: Global markets to 2029
The global market for aerospace ceramics was valued at $5.3 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 8.0% to reach $8.2 billion by the end of 2029. According to the International Energy Agency, the aviation industry was responsible for 2.5% of…
Market Insights
Innovations in access and technology secure clean water around the world
Food, water, and shelter—the basic necessities of life—are scarce for millions of people around the world. Yet even when these resources are technically obtainable, they may not be available in a format that supports healthy living. Approximately 115 million people worldwide depend on untreated surface water for their daily needs,…





