Global energy demand rose by 2.2% in 2024,1 and it is projected to increase by 15% more by 2050 as the world population grows to 10 billion.2
Because modern energy services are crucial to individual human well-being and to a country’s economic development, reliable and affordable access to electricity is paramount. However, because electricity is increasingly produced by renewable intermittent sources, such as hydro, wind, and solar,1 it is essential to develop efficient and safe electrochemical conversion and storage devices.
Today, rechargeable batteries and electrification technologies have become ubiquitous. In 2022, 91.4% of the world had access to electricity,3 and global electric car sales exceeded 17 million in 2024, reaching a sales share of more than 20%.4
A battery cell is made up of several main components (Figure 1a): anode (negative electrode), cathode (positive electrode), liquid electrolyte (ion conductor), and porous separator (electrically nonconductive, ion-permeable membrane).5 The first battery ever developed, the voltaic pile, consisted of alternating discs of zinc and silver separated by paper soaked in salt water.6 More recent battery advances follow the progress of secondary lithium-ion batteries (LiBs), with the first commercial cell produced by Sony Group Corporation in 1991.7
In modern rechargeable batteries, lithium ions shuttle internally between the anode and cathode through the porous separator during charge and discharge cycles, facilitated by the liquid electrolyte. The anode active material is graphite, which can reversibly accept and release lithium ions. The cathode is a lithium-rich ceramic oxide-based material, typically lithium iron phosphate (LiFePO4, LFP) or layered lithium metal oxides (LiMO2). In the latter case, the M encompasses single metals, such as cobalt (LiCoO2), or multiple metals in stoichiometric ratios, typically nickel, manganese, and cobalt (e.g., LiNi0.8Mn0.1Co0.1O2, NMC811). The electrolyte solution contains lithium salts such as lithium hexafluorophosphate (LiPF6) dissolved in carbonate-based solvents, for example, ethylene carbonate, ethyl methyl carbonate, or dimethyl carbonate. The separator is a polymeric membrane made from polyethylene and polypropylene.
Although LiB energy storage products are widely used in many commercial markets, a complete understanding of their hazards is still lacking; as a result, safety considerations have often been neglected.8 In 2018, the U.S. Consumer Product Safety Commission reported more than 25,000 accounts of overheating or fire incidents that occurred within a span of five years in more than 400 different consumer products powered by lithium batteries.9 More recently, specific databases have been initiated to record and track incidents related to battery energy storage systems failure,10 as well as trends occurring in aviation.11
These incidents have made the term “thermal runaway” common in current public lexicon. It refers to the phenomenon in which a LiB enters an uncontrollable, self-heating state, which can result in eventual battery failure through extremely high temperatures, violent cell venting, smoke, and fire (Figure 1b).12
Thermal runaway can be caused by internal cell failure, such as an internal short circuit, which is an unintentional electrical connection between the anode and cathode.12 Because battery manufacturing is a multistep process (Figure 1c), stringent requirements are necessary at every stage to fabricate high-quality cells. The lack of quality controls can create defects with cascading catastrophic effects that compromise the overall integrity of the cell, manifesting as internal failure during use.8
Thermal runaway can also be caused by external conditions, including electrical, mechanical, or thermal faults, or when the battery’s use is beyond the boundaries of its nominal specifications provided by the manufacturer for current, voltage, and temperature. Such usage can trigger unintended but detrimental side reactions and products.8,12 In addition, the failure of the battery management system and thermal management system, which are safety mechanisms designed to monitor and control circuitry and temperature, respectively, can lead to catastrophe.8
Hence, to better understand the electrical and thermal safety hazards, it is necessary to identify and mitigate the potential risks through scientific methods.
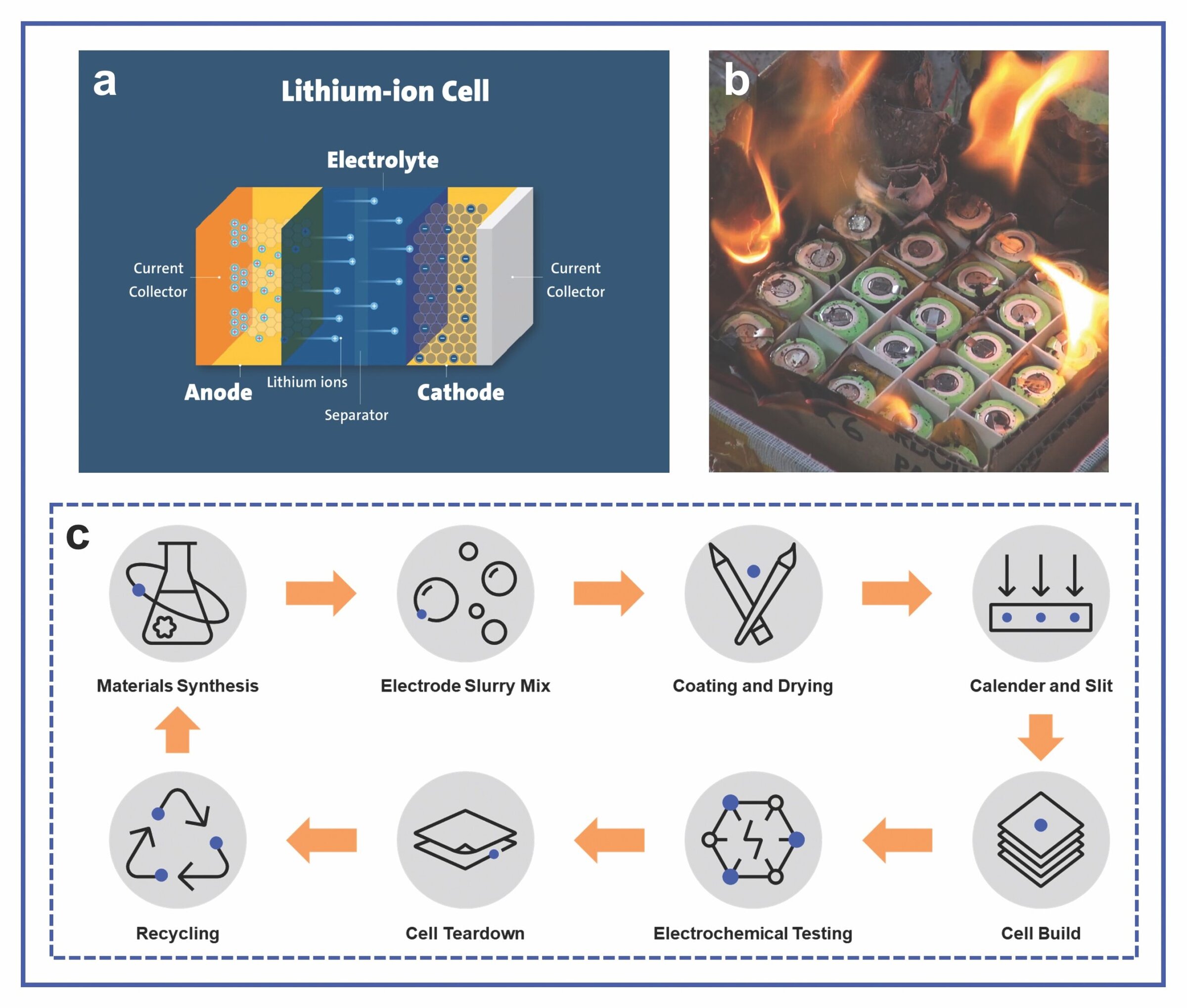
Figure 1. a) Basics of a LiB cell, b) battery fire resulting from thermal runaway, and c) LiB cell manufacturing process. Credit: UL Research Institutes
Working toward a safer world at ESRI
At the Houston-based Electrochemical Safety Research Institute (ESRI), a unit of UL Research Institutes (ULRI), we are advancing the safer design and deployment of energy storage and energy generation technologies through rigorous fundamental and applied scientific studies. Understanding the science behind the causes of safety risks in electrochemical energy provides relevant and accurate solutions that reduce or eliminate potential hazards posed by high-energy and high-power energy storage and energy generation systems.
Our discovery-driven research explores the performance and safety of energy technologies to mitigate risks.13 It centers on gathering data throughout the whole design process, from materials and cells to commercial battery packs and modules. To investigate the potential risks of current and future battery chemistries, the Novel Materials and New Energy Forms team at ESRI focuses on the research and development of both materials and low-capacity, cell-level technologies by studying new chemistries and novel systematic engineering methods related to energy storage and conversion with early-technology readiness levels through a safety lens.
Besides strong technical expertise, ESRI also has the facilities to study the performance and safety of energy-related materials. These facilities include multiple dry boxes with low water- and oxygen-controlled environments for sample manipulation, as well as instruments for various types of measurements, synthesis, cell fabrication, and testing (Figure 2).
In the race for safer batteries, the continual expansion of ESRI’s capabilities, including both equipment and talent, allows us to be on the cutting edge of safety science research and development. But what constitutes safety research at a materials level?
First, the intrinsic and fundamental thermal properties describe how materials respond to temperature. Thermal conductivity indicates the ability of a material to transfer heat. The specific heat capacity, which can be measured from differential scanning calorimetry, is the amount of thermal energy absorbed or released to raise the temperature of a unit mass of a substance by one degree Kelvin.
Second, flammability parameters describe how materials respond to an ignition source.14 Flash point is defined as the lowest temperature at which a volatile substance evaporates to form an ignitable mixture with air in the presence of an ignition source. The self-extinguishing time describes the ability of a material to cease burning after the removal of an ignition source.
Third, hazardous and gaseous species characterization allows the understanding of unfavorable products harmful to human health during the manufacturing process or from undesirable battery failure reactions. Thermogravimetric analysis elucidates a material’s weight loss as a function of temperature, and Fourier-transform infrared spectroscopy and gas chromatography with mass spectrometry identify chemical bonds and chemical species, respectively. When these techniques are coupled together, the data can provide in-depth information about the possible reaction pathways and products formed among battery materials during thermal runaway, especially when these safety science techniques are applied to simulate a full cell with combined battery components.
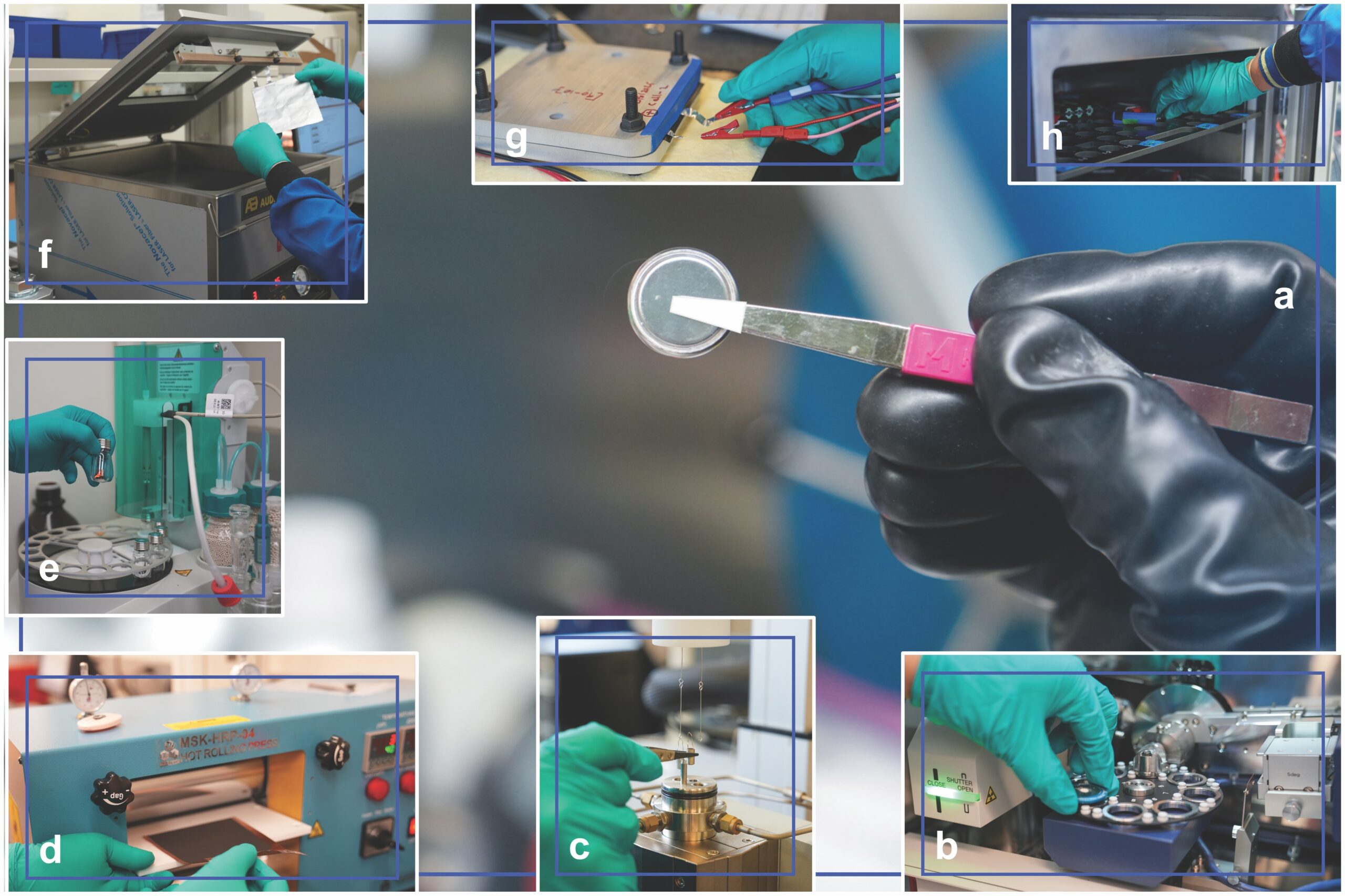
Figure 2. Some examples of the research and development capabilities at ESRI for materials performance and safety: a) in-house fabricated coin cell assembled in the dry box, b) powder X-ray diffraction sample loading, c) sample loading for thermogravimetric analysis with differential scanning calorimetry, d) calendering of in-house fabricated electrode, e) Karl Fischer moisture analysis titrator sample loading, f) vacuum sealing of in-house fabricated pouch cells, g) connecting pouch cells in pressure stacks for electrochemical testing, and h) placing an 18650 cylindrical cell in a temperature chamber for electrochemical characterization. Credit: UL Research Institutes
Innovations in lithium-ion battery technology
At ESRI, efforts to address the issue of thermal runaway in current LiB technology center around various cell components, including electrolyte additives, LiB cathode and anode active materials, and separator membrane material modifications.
Flame-retardant ions as electrolyte additives
To improve the performance of LiBs, small amounts of additives are included in the electrolyte to enhance the battery’s performance, cyclability, thermal stability, safety, and various physical properties. Because the highly volatile and flammable organic electrolyte becomes an unstable component when a LiB goes into thermal runaway, flame-retardant additives that reduce burning time and increase the electrolyte solution’s flash-point temperature can improve its safety during this phenomenon.
A project with John Protasiewicz, Hurlbut Professor of Chemistry at Case Western Reserve University (Cleveland, Ohio), focuses on the enhanced performance and safety of novel flame-retardant ions (FRIons) with fire-retardant properties as safer electrolyte additives. Electrolyte formulations with and without the presence of these FRIon salts are compared.
Other than ensuring chemical and electrochemical stability, flammability parameters are particularly important to the safety of liquid electrolytes. For example, Figure 3a shows the self-extinguishing time experiment on the baseline electrolyte sample of 1.2 M LiPF6 in an ethylene carbonate–ethyl methyl carbonate (3:7 wt.% ratio). When 1 gram of the electrolyte was ignited in open-air, the time required for the flame to extinguish was recorded: The baseline electrolyte exhibited a burning duration of approximately 40 seconds after ignition. By comparison, the modified electrolytes containing the FRIon additives showed significantly shorter flame durations due to the formation of thermally stable protective layers, which prevented the combustion of the baseline electrolyte.
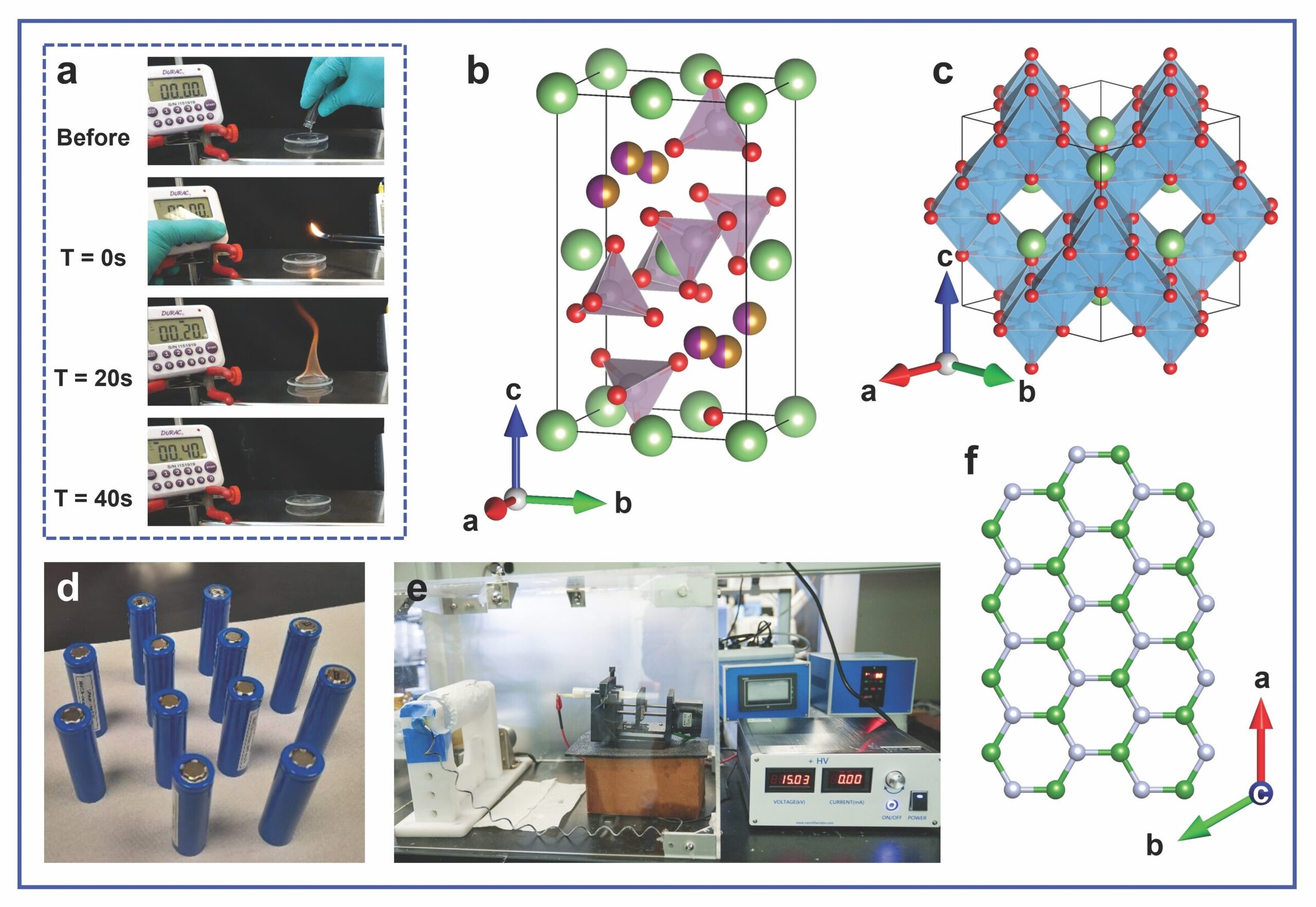
Figure 3. a) Still images taken from the self-extinguishing time experiment for electrolyte studies at different times. b) The crystal structure of orthorhombic LMFM’P (green, red, purple/gold, and lilac balls represent lithium, oxygen, manganese/iron/magnesium, and phosphorous, respectively, with lilac [PO4] tetrahedra). c) The crystal structure of cubic LTO (green, red, and blue balls represents lithium, oxygen, and titanium, respectively, with blue [TiO6] octagons). d) Lab-fabricated 18650 cylindrical cells. e) The electrospinning setup. f) The structure of an hBN layer (green and silver balls represent boron and nitrogen, respectively). Credit: Credit: a,b,c,f) UL Research Institutes; e) Xiaonan Shan; d) Palani Balaya
New lithium-ion battery electrode active materials
Battery active materials beyond the typical ones used in commercial LiBs, such as graphite anodes and LFP or LiMO2 cathodes, are also explored through the scaling-up of in-house synthesized magnesium-doped lithium manganese iron phosphate (LiMn1–x–yFexMgyPO4, LMFM’P, Figure 3b) cathode for pairing with lithium titanate (Li4Ti5O12, LTO, Figure 3c) anode. These electrodes are being used to fabricate cells similar to commercial 18650 cylindrical cells (Figure 3d) for fast-charging and high-power applications in collaboration with Palani Balaya, associate professor of mechanical engineering at the National University of Singapore.
Olivine LMFM’P, which is isostructural to LFP, has a similar theoretical specific capacity (170 mAh/g) but offers a higher voltage for lithium intercalation to achieve increased energy densities. Although spinel LTO has a lower theoretical specific capacity (175 mAh/g) than graphite, it is highly stable with zero volume expansion and contraction during the lithium interaction process; these properties allow it to handle faster charge and discharge rates for high-power-density applications.
Besides assessing the battery performance in small capacity coin cells, Balaya and his group fabricated cells in a commercial 18650 cell configuration to better understand the scale-up requirements of this chemistry pairing. Moreover, the safety of these materials and cells through thermal properties, flammability parameters, and hazardous and gaseous species characterization will be further examined.
Hexagonal-boron-nitride-doped polyvinylidene-fluoride separator membrane
The polymeric separator, which is located between the anode and cathode in a cell, plays an integral role in battery safety. Because separator failure such as puncture or shrinkage can cause internal short circuits, advances in separator materials (e.g., enhanced thermal stability, shutdown mechanisms, and flame-retardant coating) are crucial for safer battery operation.
At ESRI, research scientist Bicy Kottathodi is using electrospinning, a method to produce micro- and nanofibers from polymer solutions, to synthesize polyvinylidene-fluoride (PVDF) membranes. These porous, flexible membranes exhibit high mechanical integrity and ion transport capability (Figure 3e), making them a promising polymer separator candidate for LiBs.
In addition, hexagonal boron nitride (hBN, Figure 3f) is a thermally conductive and electrically insulating material. When incorporated into the PVDF membrane, it can improve localized heat dissipation to reduce the risk of hot spots and mitigate thermal runaway.
The aim of this study is to design a thermally stable hBN-PVDF composite separator by optimizing the electrospinning conditions and evaluating the membrane through materials characterization, electrochemical performance, and safety properties. Its enhanced wettability, ionic conductivity, flame retardancy, and mechanical and thermal stability are compared with conventional polyolefin-based separators. Alternatives beyond the lithium-ion battery paradigm Moving beyond present-day, state-of-the-art LiB research, ESRI is also investigating unique battery fabrication methods, potentially safer solid-state batteries (SSB) and magnesium-based batteries, and alternative green hydrogen production as part of a broader strategy to enable safe and sustainable energy solutions.
3D printing of anode-free solid-state batteries
SSBs are constructed by substituting the highly volatile and flammable organic electrolyte and polymer separator in conventional LiBs with a single layer of solid electrolyte, which is an ionic conductor and electrically insulating material. In addition, anode-free refers to the absence of an anode during the initial battery assembly, with the lithium-rich cathode instead being the lithium source.
Together with Zheng Fan, assistant professor of mechanical engineering technology at the University of Houston, the goal of this collaboration is to investigate 3D printing as an alternative cell manufacturing technique to fabricate viable anode-free SSBs. 3D printing or additive manufacturing is a method that can enable thinner battery layers and potentially achieve higher density cells as compared to those from conventional battery manufacturing.
The primary development of this technology is optimizing slurries for the 3D-printed solid-state electrolyte and interlayer (between the copper current collector and solid-state electrolyte), which will be paired with a tape-casted cathode for full cell electrochemical characterization. Figure 4a shows the printed interlayer using a modified 3D printer setup with a camera attachment to capture the process (Figure 4b).
Concurrently, we are also teaming up with another institute at ULRI, the Chemical Insights Research Institute (Marietta, Ga.), which specializes in the toxicological assessment of hazardous chemical emissions, to further evaluate the detection of any unfavorable and undesirable products emitted during the 3D printing process for human health safety improvements.
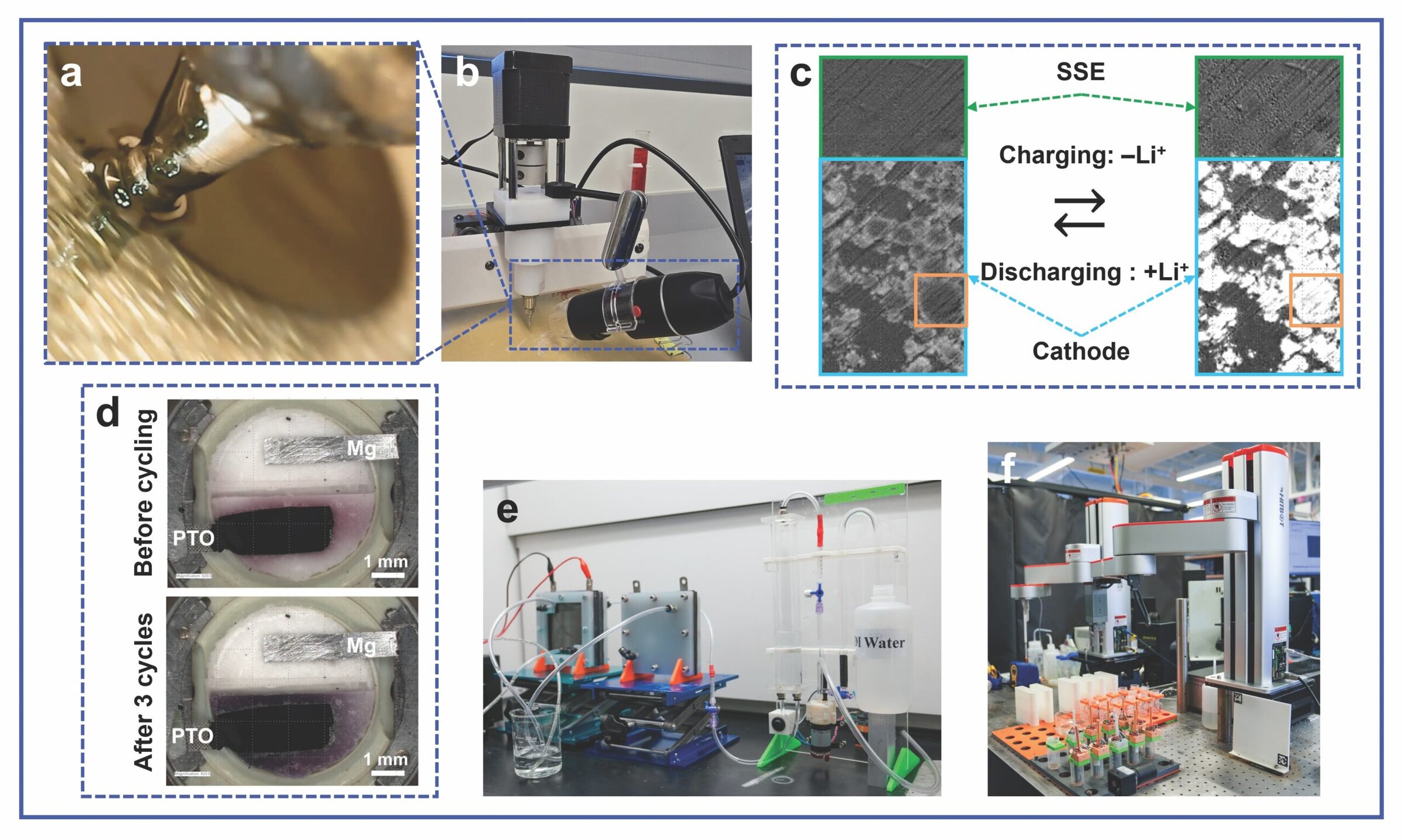
Figure 4. a) Still image of the 3D printing process. b) Modification of the 3D-printer setup with a camera attachment to capture the process in Figure 4a. c) Optical response of NMC cathode particles in a solid-state battery (Li-In anode | Li6PS5Cl electrolyte| NMC cathode) during operando microscopy. d) Operando optical characterization of a magnesium battery (Mg anode | MBF electrolyte | PTO cathode) with Mg-SPEEK separator.15 e) The electrolyzer setup for catalytic water-splitting and gas collection. f) The robotic platform. Credit: a,b) Zheng Fan; c,e,f) Xiaonan Shan; d) Reprinted with permission from Ren et al., ACS Nano.15 Copyright 2025 American Chemical Society
Operando studies of solid-state batteries
The first collaboration with Xiaonan Shan, associate professor of electrical and computer engineering at the University of Houston, revolves around understanding the performance and safety of the emerging SSB technology through operando investigations, which are critical to identifying the interfacial reactions and resistances between the battery layers during electrochemical cycling. In this study, specialized setups are developed for operando optical microscopy and Raman spectroscopy techniques. Other than room-temperature experiments, cycling at high temperature to understand the thermal behavior at the electrode–electrolyte interface will help identify thermal instabilities and failure mechanisms.
Figure 4c follows a full SSB cell undergoing electrochemical testing with two optical images zoomed in on the layers of the solid-state electrolyte (green boxes) and cathode (cyan boxes) at different states of charge. To guide the eye, the same NMC cathode particle is also highlighted in orange boxes. The left image shows the pristine cell.
During charging, as lithium ions are extracted from the NMC cathode, the optical response of the cathode particles changes: The particles get brighter, starting a gradient from the edges until the whole particle is radiating light, as shown on the right image. The opposite is observed during discharge: As the lithium ions are reinserted into the NMC, the particles become less bright. This observation illustrates that the reversible optical changes correlate well with the movement of lithium ions during cycling and, therefore, suggests brightness is a good tool to provide visualization and evidence of lithium ion diffusion and migration under various conditions.
Selective membranes for magnesium batteries
Compared to lithium technology, magnesium-based batteries have the potential to provide higher energy density and improved safety due to reduced dendrite formation. The investigations to identify, characterize, and optimize membrane separators with good magnesium-ion conductivity and selectivity for an established system (magnesium anode, magnesium alkoxyl fluorinated borate electrolyte, and pyrene-4,5,9,10-tetraone organic cathode) were successfully examined with Yan Yao, the Hugh Roy and Lillie Cranz Cullen Distinguished Professor of electrical and computer engineering at the University of Houston.
The PTO cathode can accept two stoichiometric magnesium ions to form Mg2PTO through a two-step electrochemical reaction with an intermediate cathode species, Mg1PTO, which is soluble and has a distinct purple color. This reaction allows optical microscopy to be an effective technique for studying its diffusion evolution.
Figure 4d demonstrates that the fluorine-free magnesiated sulfonated poly(ether ether ketone) (Mg-SPEEK) selective membrane facilitated magnesium ion conduction while effectively rejecting soluble organic species during full cell cycling through operando optical characterization. Because there is no bleeding of color into the magnesium anode side, Mg1PTO is effectively confined to the PTO cathode region, indicating that the Mg-SPEEK membrane was instrumental in preventing the cathode–anode crosstalk.15
Because this technology is nascent, next steps include understanding the mechanisms of the stripping-induced magnesium pulverization and evaluating the safety performance of this promising battery chemistry.
Green hydrogen generation from sea water using alternative catalysts
Hydrogen is a promising energy carrier that can effectively store and deliver energy. It is produced through various methods, though so-called “green” hydrogen is the most environmentally friendly, as it is produced by renewable energy sources with near-zero greenhouse gas emissions.
Water electrolysis is a clean process to generate green hydrogen and oxygen. Platinum metal is a typical high-performance catalyst used in the hydrogen evolution reaction cathode process. However, it is an expensive, rare, and precious metal.
This research project is ESRI’s second collaboration with University of Houston Professor Shan to develop cheaper substitutes as alternative catalysts for the hydrogen evolution reaction process to effectively generate green hydrogen during the (sea) water-splitting reaction. Figure 4e shows the lab-scale electrolyzer setup used for synthesis and characterization of the catalysts, as well as to understand the lab-based viability, scalability, and safety requirements relating to electrolysis. Because the alternative catalysts under investigation include phosphorus-based alloys with multiple metals, replacing the traditionally labor-intensive, trial-and-error experimentation process with an automatic robotic platform (Figure 4f) allows the compositions of the catalysts to be optimized within a shorter time. A video showing the different elements of this project in action can be seen below.
Documentation and database building
The impact of science and engineering lies not only in research and development but also in the essential collection and dissemination of data. At ESRI, we are ensuring that documenting safety information and database building are a part of our discovery-driven research.
Electric Vehicles for American Low-Carbon Living program
Together with Sandia National Laboratories, the University of Maryland, Purdue University, as well as six other collaborators, ESRI is a part of the Electric Vehicles for American Low-Carbon Living (EVs4ALL) program, under the Department of Energy Advanced Research Projects Agency–Energy.16
One of the aims of this program is to perform failure mode and effects analysis (FMEA) on current and prospective, precommercialized battery technologies through a combination of experimental methodology and modeling framework regarding their thermal properties, and to produce a multiscale safety testing manual report. “Failure mode” refers to the way in which something might fail, and “effects analysis” studies the results of those failures. Together, the FMEA is a structured approach to identify and assess potential failures in a system, product, or process.
In this program, ESRI is applying the risk assessment tool toward a variety of battery technologies by combining experiments, literature, and knowledge to proactively anticipate potential issues, assess their impact, and develop strategies to mitigate or prevent them. This engagement highlights the need to ensure that safety, performance, and reliability of new battery chemistries are prioritized throughout the whole development process. The FMEA document facilitates informed decision-making for stakeholders, ultimately contributing to the successful deployment and adoption of advanced electric vehicle technologies.
Library (for) Battery Research and Reference (Internal ESRI)
Although our interests lie in pushing the envelope for safer novel battery technologies, in parallel, it is also essential to produce high-quality data in our research studies through optimized processes on well-established systems as a baseline comparison for new chemistries. The Library (for) Battery Research and Reference (Internal ESRI) program, with the acronym LiBRaR(IE), is an internal project at ESRI with the goal of documenting a wide range of our laboratory standard operating procedures. This project includes
- Establishing a systematic database regarding battery materials and best practices;
- Developing and optimizing repeatable and reliable chemistry and engineering processes (e.g., electrode engineering, coin- and pouch-format cell fabrication, testing benchmarks, destructive physical analysis, design of experiments, and results analysis); and
- Generating in-house characterization data from various instruments on a commercial battery technology to serve as a reference for future studies.
Furthermore, our intention for this library goes beyond gathering data. The purpose is also to release and report the relevant information to other battery researchers through meetings (conferences and summits) and publications (peer-reviewed scientific journals and ULRI white papers).
Prospective research projects and other ESRI endeavors
ESRI is interested in pursuing new projects as part of our expanding portfolio on safety, science, and engineering, and we look forward to research and development growth in more areas. Other pending projects include various collaborations on sodium-ion battery technologies (cathodes, liquid electrolytes, solid-state electrolytes, bipolar cell design) and upcoming internal investigations committed to developing the next-generation of sulfur and SSB chemistries, chemistry-specific material- and cell-level safety characterizations, and other energy storage and conversion materials related to hydrogen.
Other than the investigations into novel materials and new energy forms, ESRI also performs research on recycling, battery and hydrogen safety, and modeling.13 It is also important to communicate our research findings outside the scientific community through outreach activities. One example of our public education and engagement initiatives is the Be Nice To Your Device campaign, which promotes safe and sustainable LiB recycling (Figure 5).
Figure 5. ESRI’s Be Nice To Your Device campaign promotes safe and sustainable LiB recycling. Learn more about the program by visiting benicetoyourdevice.org. Credit: UL Research Institutes
Driving energy research for a safe and sustainable future
As the world moves further into electrification and toward net-zero emissions, understanding the performance and safety of energy storage, conversion, and generation devices and systems is imperative.
At ESRI, our interest lies in energy safety across all points of the energy technology development process, from materials and cells to scaling commercially viable applications of lithium-ion batteries, future battery chemistries, and other renewable energy technologies. The multipronged scientific research taking place at ESRI and through various partnerships exemplifies the need to recognize the risks and drive solutions to reduce potential hazards.
Acknowledgments
The author deeply recognizes ESRI colleagues (J. A. Jeevarajan, V. R. Rikka, B. Kottathodi, T. Mullee, F. S. Gray, M. S. Ng, and M. R. Trujillo) and collaborators (P. Balaya, J. D. Protasiewicz, Z. Fan, X. Shan, and Y. Yao) for their review and suggestions.
1International Energy Agency, “Global Energy Review 2025.” Published March 2025.
2ExxonMobile, “ExxonMobil Global Outlook: Our View to 2050.” Published August 2024.
3World Bank, International Energy Agency, International Renewable Energy Agency, United Nations Statistics Division, and World Health Organization, “Tracking SDG 7: The Energy Progress Report 2024 (English).” Published 31 Dec. 2024.
4International Energy Agency, “Global EV Outlook 2025.” Published May 2025.
5UL Research Institutes, “What are lithium-ion batteries?” Published 14 Sept. 2021.
6A. Volta, “XVII. On the electricity excited by the mere contact of conducting substances of different kinds. In a letter from Mr. Alexander Volta, F. R. S. Professor of Natural Philosophy in the University of Pavia, to the Rt. Hon. Sir Joseph Banks, Bart. K.B. P. R. S.,” Philosophical Transactions of the Royal Society of London 1800, 90: 403.
7A. Dhage, “SONY Lithium-ion batteries: World’s first commercialized LiB 1991,” Battery Design. Published 12 March 2023.
8J. Lamb et al., “New developments in battery safety for large-scale systems,” MRS Bulletin 2021, 46: 395.
9U. S. Consumer Product Safety Commission, “Status report on High Energy Density Batteries Project.” Published 12 Feb. 2018.
10Electric Power Research Institute, “BESS failure incident database.” Updated 17 March 2025.
11UL Standards and Engagement, “Advancing battery safety in aviation.”
12UL Research Institutes, “What causes thermal runaway?” Published 20 Aug. 2021.
13UL Research Institutes, “Ongoing research.”
14S. Hess et al., “Flammability of Li-ion battery electrolytes: Flash point and self-extinguishing time measurements,” Journal of The Electrochemical Society 2015, 162(2): A3084.
15W. Ren et al., “Fluorine-free ion-selective membrane with enhanced Mg2+ transport for Mg-organic batteries,” ACS Nano 2025, 19(5): 5781.
16A. Bates et al., “A multi-scale framework for advancing battery safety through early calorimetric analysis of materials and components,” The Electrochemical Society Interface 2024, 33(3): 69.
*All references verified as of June 23, 2025
Cite this article
W. Si Tang, “Powering a safer future with novel energy solutions,” Am. Ceram. Soc. Bull. 2025, 104(6): 26–33.
About the Author(s)
Wan Si Tang is director of research for novel materials and new energy forms at the Electrochemical Safety Research Institute, a unit of UL Research Institutes (Houston, Texas). Contact Tang at wansi.tang@ul.org.
About the Company

Founding and future goals
Founded in 1894, UL Research Institutes (Evanston, Ill.) is a nonprofit research organization dedicated to advancing public safety through scientific discovery. From fire mitigation and air quality to safe energy storage and digital privacy, the UL Research Institutes team conducts rigorous independent research, analyzes safety data, and partners with experts to uncover and act on existing and emerging risks to human safety. Learn more about the organization and its research by visiting UL.org.
The Electrochemical Safety Research Institute (Houston, Texas), a unit of UL Research Institutes, was founded in 2015 and is led by Judy Jeevarajan, vice president and executive director. This unit specializes in advancing the safer design and deployment of energy storage and energy generation technologies. Learn more about the research being conducted at ESRI at UL.org/ESRI.
Related Articles
Market Insights
Engineered ceramics support the past, present, and future of aerospace ambitions
Engineered ceramics play key roles in aerospace applications, from structural components to protective coatings that can withstand the high-temperature, reactive environments. Perhaps the earliest success of ceramics in aerospace applications was the use of yttria-stabilized zirconia (YSZ) as thermal barrier coatings (TBCs) on nickel-based superalloys for turbine engine applications. These…
Market Insights
Aerospace ceramics: Global markets to 2029
The global market for aerospace ceramics was valued at $5.3 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 8.0% to reach $8.2 billion by the end of 2029. According to the International Energy Agency, the aviation industry was responsible for 2.5% of…
Market Insights
Innovations in access and technology secure clean water around the world
Food, water, and shelter—the basic necessities of life—are scarce for millions of people around the world. Yet even when these resources are technically obtainable, they may not be available in a format that supports healthy living. Approximately 115 million people worldwide depend on untreated surface water for their daily needs,…




